Pyelonephritis - Stanford University School of Medicine
advertisement

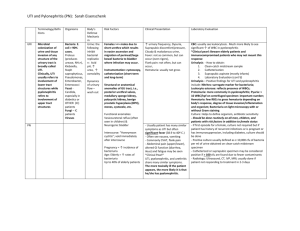
Article renal Pyelonephritis William V. Raszka, Jr, MD,* Omar Khan, MD† Author Disclosure Drs Raszka and Khan did not disclose any financial relationships Objectives After completing this article, readers should be able to: 1. Describe the epidemiology of urinary tract infections and pyelonephritis in children. 2. Discuss the risk factors for the development of pyelonephritis. 3. Compare and contrast methods to diagnose urinary tract infection and pyelonephritis in children. 4. Describe the management of pyelonephritis. 5. Explain the long-term complications of pyelonephritis. relevant to this article. Introduction The frequency of urinary tract infections (UTIs) is second only to that of respiratory tract infections in the pediatric population. UTIs often are separated into infections of the lower urinary tract that involve the bladder and urethra and those of the upper tract that involve the kidneys, renal pelvis, and ureters. Infections of the upper tract are designated pyelonephritis. A complicated UTI, whether of the upper or lower tract, usually is associated with an underlying condition that increases the likelihood of a therapeutic failure. Underlying conditions include abnormal anatomy, urologic dysfunction, the presence of an indwelling catheter, or isolation of a multiresistant organism. Epidemiology Although the prevalence of UTI has been studied in a variety of patient populations, there are fewer data regarding the prevalence of pyelonephritis due, in part, to the difficulty in distinguishing upper from lower tract disease. The prevalence of UTI is influenced by factors such as age, sex, population sample, urine collection method, testing methodologies, diagnostic criteria, and culture. Age and sex are the most important factors. In newborns, the prevalence of UTI in preterm infants (2.9%) exceeds that of term infants (0.7%). UTI is more common in preschool-age children (1% to 3%) than in school-age children (0.7% to 2.3%). During adolescence, both sexually active girls and homosexual boys are at increased risk for developing UTI. Sex has an enormous impact on the prevalence of UTI. In a retrospective populationbased study, the cumulative incidence rate during the first 6 years of life was 6.6% for girls and 1.8% for boys. In the first 3 postnatal months, UTI is more common in boys and five to ten times more common in uncircumcised boys than in circumcised boys. Thereafter, girls are far more likely to develop symptomatic UTI. The prevalence rate of UTI is 1% to 3% in girls 1 to 5 years old and 1% in school-age children. The prevalence rate in school-age boys is 0.03%. Although risk factors for the development of pyelonephritis have not been described well, risk factors for the development of UTI include a previous history of UTI, siblings who have a history of UTI, female sex (presumably because of the short female urethra), an indwelling urinary catheter, an intact prepuce in boys, and structural abnormalities of the kidneys and lower urinary tract. Up to 50% of infants may have an underlying structural or physiologic abnormality of the urinary tract detected at the time of their first UTI. Vesicoureteral reflux is the most common and important risk factor for the development of pyelonephritis. *Associate Professor of Pediatrics and Director, Infectious Disease Service, Department of Pediatrics, University of Vermont College of Medicine, Burlington, Vt. † Department of Family Medicine, Fletcher Allen Health Care, Burlington, Vt. 364 Pediatrics in Review Vol.26 No.10 October 2005 renal pyelonephritis Although many Enterobacteriaceae and other organisms may cause UTI in children, Escherichia coli is the most common pathogen. E coli can be isolated in approximately 90% of patients at the time of their initial UTI and in more than two thirds of patients who have recurrent UTI. Other organisms commonly found in patients who have community-acquired UTI include Enterobacter, Proteus, and Klebsiella sp. Streptococcus agalactiae may cause pyelonephritis in neonates. Enterococcus sp may cause up to 5% of UTIs and often is associated with more complex genitourinary tract abnormalities. Coagulase-negative staphylococci and Lactobacillus sp are rare causes of cystitis or pyelonephritis. Pathophysiology In almost all children outside the immediate newborn period, pyelonephritis develops after fecal flora colonize the urethra and ascend into the bladder and kidneys. The interval between colonization, infection, and disease is not known. Children can present with pyelonephritis without preceding symptoms typical of lower tract infection. Several virulence factors have been identified in organisms that cause pyelonephritis. A small number of E coli O serotypes are responsible for approximately 80% of cases of pyelonephritis, 60% of cystitis, and 30% of asymptomatic bacteriuria but are found in only 30% of fecal flora. The most important virulence factors, particularly in patients whose urinary tracts are normal, govern adherence or attachment to host mucosal cells. Adhesins, molecules mediating attachment, are on the surfaces of bacteria or bacterial appendages. Three major adhesins associated with strains of E coli causing UTI are PAP, AFA, and SFA. PAP is the best known and most closely associated with pyelonephritis. This molecule is located on the P pili or fimbriae and recognizes P blood group determinants found on cells lining the urinary tract. Operons that control expression of PAP, AFA, and SFA molecules are found in most E coli isolated from patients who have pyelonephritis. Host defenses against the development of UTI relate primarily to anatomic and physiologic considerations. The ability to empty the bladder of urine regularly and completely is the most important host defense mechanism against infection. Patients who do not completely empty the bladder, regardless of the reason, are at signif- icantly greater risk for the development of a bladder infection. The most important risk factor for the development of pyelonephritis in children is vesicoureteral reflux (VUR). VUR is detected in approximately 10% to 45% of young children who have symptomatic UTI. Although there is some debate about how reflux damages the renal parenchyma, refluxed infected urine from the bladder increases the risk of pyelonephritis and, in young children, renal complications such as scarring. Clinical Findings The clinical manifestations of pyelonephritis are highly variable. Older children may present with fever, chills, nausea, and flank pain in addition to typical signs of lower tract disease such as dysuria, urgency, and increased urinary frequency. Clinical findings include suprapubic and costovertebral (CVA) tenderness. Flank pain, fever, and vomiting occur more commonly in patients who have pyelonephritis than in those who have lower tract Fever and irritability are the most common presenting findings in infants who have pyelonephritis. disease, but these finding are neither specific nor sensitive for pyelonephritis. As many as 25% of children who have no classic signs or symptoms of pyelonephritis are ultimately confirmed to have upper tract disease; up to 50% of children who have flank pain have no other evidence of pyelonephritis. Fever and irritability are the most common presenting findings in infants who have pyelonephritis. Other findings include poor feeding, lethargy, and in toddlers, abdominal pain. Traditionally, pyelonephritis is suspected in any infant or child who has fever, emesis, flank pain, or CVA tenderness on physical examination and a positive urine culture. Diagnosis UTI should be considered in any child 2 months to 2 years of age or younger who has unexplained fever. Approximately 5% to 7% of febrile infants younger than 8 weeks of age have a UTI compared with approximately 5% of febrile children younger than 1 year of age and 2% Pediatrics in Review Vol.26 No.10 October 2005 365 renal pyelonephritis Sensitivity and Specificity Ranges for Components of the Urinalysis in the Diagnosis of Urinary Tract Infection Table. Test Leukocyte esterase White blood cells on microscopy Nitrite Bacteria on microscopy Sensitivity (%) (range) Specificity (%) (range) 70 to 95 30 to 100 65 to 90 45 to 100 15 to 80 15 to 100 90 to 100 10 to 100 of all febrile children younger than 5 years of age. Patients usually are diagnosed following examination of their urine. The purpose of the urinalysis is to identify those children at greater or lesser risk of having a UTI. Culture of an appropriately obtained urine specimen is the laboratory test that establishes or excludes the presence of UTI. Urine can be collected by bag technique, mid-stream clean catch, urethral catheterization, or suprapubic aspiration. Although many physicians continue to rely on bagged urine specimens, UTI should not be diagnosed by a bagged specimen because of its low specificity and poor positive predictive value. A negative culture from an appropriately obtained bagged specimen, however, may be useful in some situations to exclude UTI. For a child 2 months to 2 years of age, urine for culture should be obtained by suprapubic aspiration or transurethral catheterization. Both of these methods are simple and easy to perform and give reliable results. In older children, midstream clean catch of spontaneously voided urine after appropriate cleansing of the urinary meatus is acceptable. A clean urine specimen can be used to assess for evidence of inflammation or bacteria. The leukocyte esterase test identifies an enzyme produced by white blood cells. A positive leukocyte esterase test usually corresponds to at least 5 white blood cells per highpower field, with a sensitivity of 70% to 95% (Table). Direct visualization of the unspun urine gives information about the number and characteristics of white blood cells. More than 5 white blood cells per high-power field represents pyuria. Similar to the leukocyte esterase test, the presence of white blood cells in the urine is a sensitive but nonspecific test for UTI. The exception is the finding of white blood cell casts, which, in the absence of signif366 Pediatrics in Review Vol.26 No.10 October 2005 icant hematuria, usually is diagnostic of pyelonephritis. Some laboratories perform an “enhanced urinalysis,” which involves examining a drop of urine on a hemocytometer. The presence of more than 10 white blood cells suggests a UTI. Many gram-negative organisms reduce nitrates to nitrites in the urine. A positive nitrite test is an insensitive but relatively specific test for UTI (Table). The presence of bacteria in a spun urine sample is neither specific nor sensitive enough to use clinically. Although laboratories rarely perform routine Gram stains on urine, a positive Gram stain result on an unspun urine specimen is diagnostic of UTI. Many children who have radiographically confirmed pyelonephritis have elevated peripheral white blood cell counts or nonspecific markers of inflammation such as an increased erythrocyte sedimentation rate or C-reactive protein value, but these laboratory tests are neither sensitive nor specific enough to confirm or exclude pyelonephritis. The diagnosis of UTI is confirmed by the growth of a significant number of bacteria in a properly obtained urine specimen. Based on studies of healthy women more than 50 years ago, growth of more than 105 organisms/ mL of voided midstream urine is indicative of UTI. Specimens that grow fewer than 104 organisms are unlikely to represent UTI. In the appropriate clinical setting, growth of 104 and 105 organisms may represent UTI. Any growth of gram-negative bacteria in urine obtained by suprapubic aspiration indicates a UTI. For children in whom urine is obtained by transurethral catheterization, some have proposed that more than 50,000 colonies/mL of urine be used as the criterion for diagnosing the infection. Urine cultures do not distinguish between lower and upper urinary tract infection Approximately 10% to 20% of adult women who have pyelonephritis have concomitant bacteremia. In children, the prevalence of bacteremia in patients younger than 2 months of age diagnosed as having a UTI is 22.7% and in patients between the ages of 2 months and 36 months of age is 3.0%. Infants and children whose urine and blood cultures are positive are assumed to have pyelonephritis. Imaging studies are needed infrequently to confirm a diagnosis of pyelonephritis. Computed tomography scans are more sensitive than ultrasonography at detecting changes consistent with pyelonephritis, but they require the use of intravenous contrast. DMSA renal scintography is the imaging study of choice to confirm or exclude the diagnosis of pyelonephritis as well as to detect renal scarring. Its routine use, however, is limited renal pyelonephritis to situations such as the challenging patient who has a neurogenic bladder or significant genitourinary abnormalities. Treatment Various antimicrobial agents are useful for the treatment of UTI. Antimicrobial selection and route of administration are guided by local microbial resistance patterns and the ability of the patient to take and retain oral agents. Many commonly used antibiotics are concentrated in the urine and are useful in treating lower tract disease. Renal parenchymal antibiotic levels, however, more closely resemble bloodstream levels. The consequence is that nitrofurantoin, which achieves negligible levels in the blood but is concentrated in the urine, is a good drug for the treatment or prophylaxis of lower UTI but not for pyelonephritis. Children who are suspected of having pyelonephritis are started empirically on antibiotics effective against the usual pathogens for their clinical condition. Children antibiotics had no advantage over oral antibiotics regarding time to defervescence, renal scarring, duration of hospitalization, and time to urine sterilization. Many oral agents can be used for the empiric therapy of pyelonephritis in patients well enough to take oral medications. Amoxicillin, however, is no longer an acceptable choice because approximately 40% of E coli strains elaborate a penicillinase. TMP-SMX has been a standard first-line oral agent for some time and may be more effective than beta-lactams at treating UTIs. However, E coli resistance to TMP-SMX is becoming more common. TMP-SMX should not be used for empiric therapy of pyelonephritis if local resistance to the drug in E coli exceeds 10% to 20%. Alternatives to TMPSMX include first-, second-, and third-generation oral cephalosporins, penicillin/beta-lactamase inhibitor combinations, and for the child older than 12 months of age, ciprofloxacin. Because of concerns about adverse effects of ciprofloxacin in young children, when it appears to be the best drug, the potential benefits and problems associated with it should be discussed with the parents. For patients who have a confirmed UTI, antimicrobial susceptibility testing results should guide therapy. Whenever possible, amoxicillin or TMP-SMX should be used. Although few randomized trials comparing treatment regimens for pyelonephritis in children have been performed, the available evidence suggests that 10 to 14 days of antimicrobial therapy is adequate for uncomplicated pyelonephritis. After completion of 10 to 14 days of antibiotics for their initial UTI, children ages 2 months to 2 years should be continued on prophylactic antibiotics until imaging studies are completed and VUR is excluded. Follow-up urine culture after 24 hours of therapy is not indicated because it rarely is positive. Almost 90% of infants who have febrile UTIs and presumed pyelonephritis were afebrile within 48 hours of initiation of antibiotics. Those who remained febrile for more than 48 hours did not have any different laboratory or clinical findings from those who became afebrile in fewer than 48 hours. Most important, there was not a higher rate of complications in those who failed to respond in 48 hours. However, many clinicians re-evaluate children who have not become afebrile within 48 hours with another urine culture or an imaging study, looking for evidence of urinary tract obstruction. For patients who have a confirmed UTI, antimicrobial susceptibility testing results should guide therapy. deemed toxic or ill enough to require hospitalization usually are started on intravenous antibiotics. Ampicillin and gentamicin have been used empirically for decades to treat pyelonephritis. Gentamicin is effective against most aerobic gram-negative bacilli; ampicillin is the drug of choice for enterococci. Alternatives to this regimen include most first-, second-, or third-generation cephalosporins, trimethoprim-sulfamethoxazole (TMP-SMX), and penicillin/beta-lactamase inhibitor combination drugs. Parenteral antibiotics most often are continued until the patient is afebrile for 24 hours. For children who are presumed to have pyelonephritis and who appear ill or are vomiting but are not ill enough to require hospitalization, an intramuscular dose of ceftriaxone or gentamicin can be administered followed by oral antibiotics. For children older than 1 month of age who are suspected of having pyelonephritis, do not appear toxic, and can take oral agents, oral antimicrobial agents can be started. At least one study has shown that in children older than 2 months of age, intravenous Pediatrics in Review Vol.26 No.10 October 2005 367 renal pyelonephritis ferred choice for all boys of any age at the time of their first UTI. Radionuclide cystography often is performed in girls and for follow-up VCUGs. Unfortunately, it is uncertain whether the identification and treatment of children who have primary VUR confer clinically important benefits. In randomized, controlled trials, no treatment or treatment of VUR with long-term antibiotics, surgery, or a combination of antibiotic prophylaxis and surgery seemed to have similar results. Natural history studies suggest that long-term medical management is as effective as surgical therapy for those who have primary VUR. Currently, children who have VUR are placed on long-term prophylactic antibiotics such as daily nitrofurantoin, trimethoprim, or TMP-SMX. Children who have VUR and recurrent UTI with progressive renal damage despite prophylactic antibiotics or those who have severe reflux often are considered for surgical correction of the VUR. Complications Figure. Fluoroscopic VCUG showing grade III reflux. Prevention The goal of medical management of patients who have pyelonephritis not only is to eradicate the infection but to identify those patients at risk for subsequent infection and renal scarring. The American Academy of Pediatrics (AAP) recommends that children younger than age 2 years diagnosed with their first UTI be evaluated for evidence of urologic abnormalities. Many clinicians also evaluate older children who have febrile UTIs or pyelonephritis. Although one study suggested that the results of ultrasonography had no impact on the management of a select group of children who had an initial UTI, the AAP recommends that renal ultrasonography be used to define the anatomy of the urinary tract because the test is noninvasive, involves no radiation, and can define renal anatomy readily (eg, obstruction, major scarring, cysts, or renal dysplasia). Children who have UTI diagnosed in the first 2 years after birth should be evaluated with voiding cystourethrography (VCUG). Both fluoroscopic or contrast VCUG and radionuclide cystography are good at detecting reflux and duplicated systems. Fluoroscopic VCUG can provide enough information to grade reflux (Figure) according to an international scale that calculates the height of the reflux and the associated anatomic changes to the kidney and identifies bladder and urethral abnormalities better than radionuclide studies. It is the pre368 Pediatrics in Review Vol.26 No.10 October 2005 Patients who have pyelonephritis may develop bacteremia. The likelihood of developing bacteremia depends on the age of the child. Children younger than 2 months of age are the most likely to have bacteremia at the time of presentation. No laboratory test or physical findings can distinguish between patients who have bacteremia and those who do not. Children who have bacteremia have similar clinical courses as those who do not, whether treated with oral or intravenous antibiotics. Long-term complications of pyelonephritis include recurrence, renal scarring, and hypertension. Approximately 18% of boys who have a UTI before age 1 year develop recurrent infections compared with 32% when the initial infection happened after the first postnatal year. Most recurrences occur within the first year of infection. Similar to boys, girls who have their first UTI at a later age have a higher recurrence rate than those diagnosed in the first year (40% versus 26%). Each prior infection increases the risk of a recurrence by as much as 25%. Children who have pyelonephritis may develop renal scarring. For most children who have UTI in whom renal scars are found, the scars are visible on the first set of imaging studies. These original scars remain unchanged irrespective of the child’s future clinical course. Infants and young children are more prone to scarring than are children older than age 5 years and adults, although the reason is unclear. Children treated for UTI who have evidence of VUR are significantly more likely to develop scars than are children who do not have VUR. Almost all new scars in children who have VUR occur before the age renal pyelonephritis of 6 years and almost always in association with new UTIs. More severe reflux is associated with a greater risk of scarring. A long-term follow-up study of children who had grade III or IV reflux managed either medically or surgically found that the incidence of new scar formation over a 5-year period was 20% in children younger than age 2 years, 10% in children between 2 and 4 years, and 5% in children older than age 4 years at the time of enrollment. Only two children developed new scars more than 5 years after enrollment. Early detection of pyelonephritis is critical to preservation of renal function. In a recent study of United States children who were identified as having UTI early in life, only one child had renal scarring on entry. Other United States investigators also have found a low incidence of renal scarring in children who had presumed pyelonephritis identified early in life. This contrasts with older studies, which demonstrated a high incidence of scarring. Long-term follow-up studies have linked renal scarring with hypertension, decreased glomerular filtration, and end-stage renal disease. Unfortunately, the exact mechanism of risk of eventually developing hypertension by patients who have scars is not known. Moreover, it is unclear why some children who have extensive scars never develop hypertension. Currently, the best evidence suggests that children who have pyelonephritis and renal scarring be followed carefully over time. Conclusion Pyelonephritis in the pediatric patient is a serious illness. Although some aspects of the management of this disease are still open to debate, there is broad agreement that prompt recognition and treatment are essential to prevent long-term sequelae. Suggested Reading American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843– 852 Chon CH, Lai FC, Shortliffe LM. Pediatric urinary tract infections. Pediatr Clin North Am. 2001;48:1441–1459 Edelmann CM Jr, Ogwo JE, Fine BP, Martinez AB. The prevalence of bacteriuria in full-term and premature newborn infants. J Pediatr. 1973;82:125–132 Herr SM, Wald ER, Pitetti RD, Choi SS. Enhanced urinalysis improves identification of febrile infants ages 60 days and younger at low risk for serious bacterial illness. Pediatrics. 2001; 108:866 – 871 Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195–202 Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109:e70. Available at: http://pediatrics.aappublications.org/cgi/content/full/ 109/5/e70 Kraus SJ. Genitourinary imaging in children. Pediatr Clin North Am. 2001;48:1381–1424 Le Bouguenec C, Lalioui L, du Merle L, et al. Characterization of AfaE adhesins produced by extraintestinal and intestinal human Escherichia coli isolates: PCR assays for detection of Afa adhesins that do or do not recognize Dr blood group antigens. J Clin Microbiol. 2001;39:1738 –1745 Olbing H, Smellie JM, Jodal U, Lax H. New renal scars in children with severe VUR: a 10-year study of randomized treatment. Pediatr Nephrol. 2003;11:1128 –1131 Pitetti RD, Choi S. Utility of blood cultures in febrile children with UTI. Am J Emerg Med. 2002;20:271–274 Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113(suppl 1A):14S–19S Wheeler DM, Vimalachandra D, Hodson EM, Roy LP, Smith GH, Craig JC. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2004:CD001532 Wiswell T. The prepuce, urinary tract infections, and the consequences. Pediatrics. 2000;105:860 – 862 Multimedia Links AAP Guidelines on the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Available at: http://www.aap.org/policy/ac9830.htm The NIH/NIDDK Web site on pyelonephritis. Available at: http:// www.niddk.nih.gov/health/kidney/summary/pyelonep/ pyelonep.htm MEDLINEplus medical encyclopedia on pyelonephritis. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000522. htm The Atlas of Renal Pathology. Available at: http://ajkd. wbsaunders.com/atlas/35/2/atlas35_2.htm Pediatrics in Review Vol.26 No.10 October 2005 369 renal pyelonephritis PIR Quiz Quiz also available online at www.pedsinreview.org. 9. Which of the following findings or conditions increases the risk of developing a urinary tract infection (UTI)? A. B. C. D. E. Acidification of the urine. Dilution of the urine. High specific gravity. Incomplete bladder emptying. Large bladder capacity. 10. An 18-month-old boy presents with fever for 3 days. Urine is obtained by catheterization. Which of the following findings in the urine confirms the diagnosis of UTI? A. B. C. D. E. Positive Gram stain of unspun urine. Positive leukocyte esterase test. Positive nitrite test. Presence of bacteria in spun urine. Presence of 10 white blood cells in unspun urine. 11. A 15-month-old girl who has recurrent UTIs presents with fever and irritability. Analysis of her urine shows more than 50 white blood cells per high-power field. Which of the following oral medications is least likely to be effective for the empiric treatment of her presumed UTI? A. B. C. D. E. Amoxicillin. Cefuroxime. Cephalexin. Ciprofloxacin. Trimethoprim-sulfamethoxazole. 12. A 20-month-old patient is diagnosed as having her first UTI. After 24 hours of antibiotics, she is afebrile. Renal ultrasonography findings are normal. Which of the following tests should be performed on this girl? A. B. C. D. E. DMSA renal scintography. Immediate repeat urine culture. Intravenous pyelography. Repeat renal ultrasonography in 6 weeks. Voiding cystourethrography. 370 Pediatrics in Review Vol.26 No.10 October 2005