Microbial metabolism and biochemical assays
advertisement

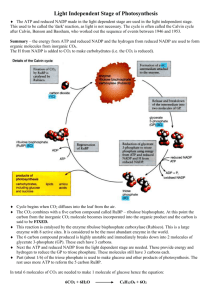
Microbial metabolism and biochemical assays By Dr. C. Rexach Microbiology Mt San Antonio College Metabolism • Sum total of all chemical reactions in living organisms • Two general types – Anabolism: building bonds,capturing energy – Catabolism: breaking bonds, releasing energy • Coupled reactions • Enzymes=biological catalysts Characteristics of enzymes • Almost all enzymes are proteins – Exception: ribozymes • Enzymes can only speed up reactions that would occur anyway • Enzymes are able to work at biological temperatures • Enzymes are sensitive to certain conditions – Remember: functional proteins work on the basis of their 3-D shape • Enzymes can be regulated Enzymes speed up reactions by reducing activation energy Enzyme components • Some enzymes require non-protein cofactors or coenzymes • Cofactors – Usually metal ions cofactor – Ca++, Mg++, etc. – Help form bridge between enzyme and substrate • Coenzymes – NAD, FAD, CoA, etc. enzyme Mechanism of enzyme action enzyme enzyme substrate product Enzyme-substrate complex Enzyme can be reused Factors influencing activity • • • • • • Temperature pH Amount of substrate Amount of enzyme Competitive inhibition Feedback inhibition Enzymes can be denatured by pH and temperature Competitive inhibition Feedback inhibition Energy production • Biochemical pathway – Sequence of enzyme catalyzed chemical reactions in cell • Oxi-redux reactions – Electrons pulled off and passed along in series of reactions – Oxidation = removal of one or more electrons from substance (often along with a H+) – Reduction = substance gains one or more electrons Oxidation-Reduction Rxns Oxidation-Reduction Rxns • In biological systems, the electrons are often associated with hydrogen atoms. • Biological oxidations are often dehydrogenations. Carbohydrate catabolism • Oxidation of carbohydrates = one of primary energy sources in cell • Most common = glucose • Two most frequently used methods – Cellular respiration • Complete breakdown of glucose into H2O, CO2 and energy • Four steps: glycolysis, intermediate step, Krebs cycle, ETS – Fermentation • Partial breakdown into lactic acid or ethanol and CO2 Note: Bacteria have many different pathways for carbohydrate metabolism based on the enzymes they are able to produce. Glycolysis = Embden-Meyerhof pathway • Overview – Begin with 1 mole of glucose = C6H12O6 – Series of enzyme mediated reactions result in formation of 2 moles of pyruvic acid (3C) and energy transfer molecules • 4ATP (2 net) • 2 NADH Summary: 4ATP-2ATP = 2ATP net 2NADH Glycolysis glucose Glucose-6-phosphate ATP Fructose-1,6 bisphosphate ATP Dihydroxyacetone phosphate Glyceraldehyde phosphate NADH 2 1,3 bisphosphoglycerate 2 ATP 2 Phosphoenolpyruvate (PEP) 2 2 ATP 2 pyruvic acid 3-phosphoglycerate Entner-Doudoroff Pathway • Each step in glycolysis is enzyme mediated • Phosphofructokinase is an enzyme which phosphorylates fructose-6-phosphate, producing fructose 1,6 bisphosphate Glucose-6-phosphate Fructose-1,6 bisphosphate phosphofructokinase • If organisms lack this enzyme, they can’t progress down Embden-Meyerhof pathway • Entner-Doudoroff pathway provides alternative way to go from glucose-6-phosphate to pyruvic acid Entner-Doudoroff Pathway • Independent of glycolysis • Produces NADPH & ATP • Two key enzymes – 6-phosphogluconate dehydrogenase – 2-keto-3deoxyglucosephosphate aldolase • Absent in gram-positive bacteria • Found in some gram negative bacteria, such as Pseudomonas, Rhizobium,Agrobacterium, Zymomonas, etc. Glucose ATP ADP Glucose-6-phosphate NADP+ NADPH 6-phosphogluconic acid 2-keto-3-deoxygluconic acid 6-phosphate pyruvate Glyceraldehyde 3-phosphate glycolysis pyruvate ATP ATP Pentose phosphate pathway • Major uses – 1. generate pentoses from hexoses – 2. generate hexoses from pentoses (gluconeogenesis) – 3. break down pentoses as a source of cellular energy • Produces acetate and pyruvate – 4. generate NADPH • Important coenzyme used by cells for reductive biosynthesis – 5. generates sugar diversity • Produces a variety of sugar derivatives in ancillary reactions • Key intermediate = ribulose-5-phosphate – Source of ribose and deoxyribose for nucleic acid production Aerobic respiration • More ATP produced by oxidative phosphorylation • Final electron acceptor is inorganic = O2 • Results in complete catabolism of glucose • Three steps – Intermediate step – Krebs cycle – Electron Transport System (ETS) Intermediate step GLYCOLYSIS Summary: 2 NADH 2 CO2 2 Pyruvic acid 2 NADH 2 CO2 2 acetyl CoA KREBS CYCLE Krebs Cycle Summary: 6 NADH 2 FADH2 2 ATP 4 CO2 Electron transport system • Electrons from NADH and FADH2 passed along series of carrier molecules embedded in cristae (eukaryotes) or plasma membrane (prokaryotes) • 3 types of carrier molecules – Flavoproteins – Cytochromes – ubiquinones • Energy released drives generation of ATP via chemiosmosis Electron Transport System FMN NADH NADH = 3ATP FADH2 = 2ATP Fe-S Q Cyt b Fe-S Fe-S Cyt c1 FADH2 Cyt c Cyt a Cyt a3 ½ O2 Chemiosmosis generates ATP Chemiosmosis Grand total for aerobic cellular respiration step #ATP Glycolysis #NADH/FADH2 #CO2 prod end products 2ATP net 2 NADH 0 CO2 2 pyruvic acid Intermediate Step 0 ATP 2 NADH 2 CO2 2 acetyl CoA Krebs Cycle 2 ATP 6NADH/2FADH2 4 CO2 ETS 34 ATP 0 H2O & CO2 0 Grand total = 38 ATP (prokaryotes) or 36 ATP (eukaryotes) 0 Without oxygen: fermentation • Final electron acceptor is organic = pyruvic acid • Anaerobic respiration: less ATP produced • Results Lactic acid Lactic Acid Fermentation: causes food spoilage, production of yogurt, pickles, sauerkraut Examples: Lactobacillus, Streptococcus Ethanol + CO2 Alcohol fermentation: Many bacteria and yeasts Examples: Saccharomyces Summary for fermentation • No new electron transfer molecules (either NADH,FADH2, or ATP) produced in intermediate step • The electrons from the 2NADH made during glycolysis are removed and transferred to pyruvic acid, the final electron acceptor. Therefore, they are unavailable for making more ATP in the ETS. • If lactic acid is end product, no CO2 is produced during fermentation • If ethanol is the end product, 2 CO2 are produced during fermentation • The total ATP produced net in fermentation = 2 Homolactic vs. heterolactic fermentation • Two types of lactic acid fermentation – Homolactic fermentation • Produces only lactic acid using pyruvic acid • Usually begins with Embden-Meyerhof pathway • Characteristic of Streptococci and some Lactobacilli – Heterolactic fermentation • Produces lactic acid, ethanol and CO2 using pyruvic acid and acetate • Begins with the pentose phosphate pathway • Characteristic of some Lactobacilli and Leuconostoc Fermentation in enteric bacteria • Type and proportion of products of anaerobic fermentation used to separate enteric bacteria into various genera • Two major patterns – Mixed-Acid Fermentation • Produces acetic, lactic, and succinic acid • Also produces ethanol and CO2 and H2 • CO2 and H2 are produced in equal amounts – 2,3 butanediol fermentation • Major products are butanediol, ethanol, CO2, and H2 • Much more CO2 is produced than H2 • Also produces small amounts of succinic, lactic, and acetic acids Mixed-Acid Fermentation glycolysis Pyruvic acid Lactic acid CO2 • CO2 is produced only from formic acid via formate hydrogen lyase • HCOOH Succinic acid Ethanol Acetyl CoA Acetic acid H2 + CO2 CO2 • Therefore, equal amounts of H2 & CO2 Formic acid H2 2,3 butanediol fermentation 2,3 butanediol + CO2 ethanol glycolysis Pyruvic acid Lactic acid Succinic acid • Produce CO2 from formic acid and from formation of butanediol Acetic acid CO2 + H2 Fermentation in microbes Anaerobic Respiration • The final electron acceptor in the electron transport chain is not O2. • Yields less energy than aerobic respiration because only part of the Krebs cycles operations under anaerobic conditions. Electron acceptor Products NO3– NO2–, N2 + H2O SO4– H2S + H2O CO32 – CH4 + H2O Lipid Catabolism Protein Catabolism Protein Extracellular proteases Amino Acids Deamination, decarboxylation, dehydrogenation Organic acids Krebs cycle Photosynthesis Figure 4.15 Photosynthesis • Photo: Conversion of light energy into chemical energy (ATP) – Light-dependent (light) reactions • Synthesis: Fixing carbon into organic molecules – Light-independent (dark) reaction, Calvin-Benson cycle Photosynthesis • Oxygenic: 6 CO2 + 12 H2O + Light energy C6H12O6 + 6 H2O + 6 O2 • Anoxygenic: CO2 + 2 H2S + Light energy [CH2O] + H2O + 2 S0 Cyclic Photophosphorylation Noncyclic Photophosphorylation • Halobacterium uses bacteriorhodopsin, not chlorophyll, to generate electrons for a chemiosmotic proton pump. Nutritional classification Photoautotrophs Source of energy = light Carbon source = CO2 Photoheterotrophs Source of energy = light Carbon source = organic Chemoautotrophs Source of energy = reduced inorganic compounds Carbon source = CO2 Chemoheterotrophs Source of energy and carbon = glucose saprophytes (decaying matter), parasites (living matter) Metabolic Diversity Among Organisms Nutritional type Energy source Carbon source Example Photoautotroph Light CO2 Oxygenic: Cyanobacteria, plants Anoxygenic: Green, purple bacteria Photoheterotroph Light Organic compounds Green, purple nonsulfur bacteria Chemoautotroph Chemical CO2 Iron-oxidizing bacteria Chemoheterotroph Chemical Organic compounds Fermentative bacteria, Animals, protozoa, fungi, bacteria. Polysaccharide biosynthesis Lipid Biosynthesis Amino Acid & Protein Biosynthesis Transamination Biosynthesis of purines and pyrimidines Biochemical tests are used to ID bacteria Carbohydrate fermentation • Investigates ability of particular bacterium to metabolize specific sugars and determines method they use • Phenol red used as pH indicator • Durham tube captures gas • Results – A, AG, AGR, negative Carbohydrate fermentation MR-VP Medium • Medium = glucose broth + peptone & dipotassium phosphate • Used to differentiate gram neg enteric bacteria • Two tests in one – Mixed acid fermentation • Results = methyl red added to determine pH change • Durham tube used to visualize gas production – 2,3 butanediol fermentation • Voges-Proskauer test • Gram negative enterics which do not use mixed-acid fermentation sometimes produce 2,3 butanediol • Add Barritt’s reagent to convert butanediol to acetoin • Pink to red color change after 30 minute incubation is positive Citrate Test • Citrate in media is only source of oxidizable carbohydrate – Citrate split to produce oxaloacetate + pyruvate – Products fermented – Also contains ammonium salts as nitrogen source • pH indicator called Brom thymol blue – Color change when citrate is used due to production of ammonia, which makes pH alkaline Citrate test + - Nitrate reduction tests • Used to detect gram negative rods • Nitrate is final electron acceptor in anaerobic respiration, reducing nitrate to nitrite • Durham tube for gas, reagents used to determine presence of nitrite • Negative tests are double-checked with Zinc dust Nitrate reduction test Catalase tests • H2O2 produced as by-product of aerobic respiration using oxygen • Protect themselves against oxidation by producing catalase • Produced by aerobes + facultative anaerobes, but not by obligate anaerobes • Test by adding H2O2 to cells on a glass slide and watching for bubbles Catalase test Indole production • Some bacteria can cleave amino acid tryptophan to prod indole + pyruvic acid • Presence of indole detected by Kovac’s reagent • Forms pinkish red layer on surface Indole Urea hydrolysis • Produced when protein and nucleic acids broken down • Organisms able to make urease convert urea to ammonia and CO2 • Ammonia becomes ammonium hydroxide in water • pH increases • Phenol red indicator used to detect change Urea hydrolysis Phenylalanine deamination • Differentiates some gram negative organisms • Oxidative deamination of phenylalanine catalyzed by phenylalanine deaminase • Detects presence of enzyme by adding 10% ferric chloride Kligler’s Iron Agar • Differentiates gram negative enterics • Multiple test medium – Fermentation of glucose and lactose – Production of H2S from cysteine catabolism • Phenol red Kligler’s Iron Agar Litmus Milk API 20E