Experiment #10 – Properties of Carboxylic Acids and Esters
advertisement

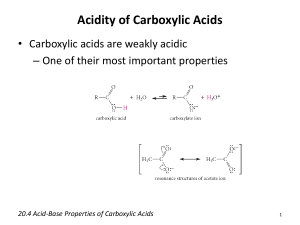
Experiment #10 – Properties of Carboxylic Acids and Esters Introduction Carboxylic acids are characterized by the carboxyl group which combines the carbonyl group of aldehydes and ketones with the hydroxyl group of alcohols and phenols. Since the carbonyl and hydroxyl groups are directly bonded to each other each affects the properties of the other. The result is that the hydroxyl group of a carboxylic acid is considerably, but not completely, different from its alcohol or phenol sibling; the same can be said when one compares the carbonyl of a carboxylic acid with that of an aldehyde or ketone. O R C O H A carboxylic acid. R may be H, alkyl, or aromatic. Just as phenols are typically 100,000 to a million times more acidic than alcohols, carboxylic acids are typically 100,000 to a million times more acidic than phenols. Nevertheless, most carboxylic acids are considered to be relatively weak acids. For example, acetic acid (R = CH3), ionizes to an extent of less than 1% when dissolved in water. When a carboxylic acid ionizes in water it donates the proton of the -OH group to a water molecule; in other words, it is a proton donor and, therefore, an acid in the Bronsted-Lowry sense. The water that accepted the proton is a base in the Bronsted-Lowry sense. The water becomes a hydronium ion and the carboxylic acid becomes a carboxylate ion in the process, as shown in the reaction below. The expression that defines the equilibrium constant for this reaction is shown below. When one realizes that in dilute aqueous solutions the concentration of water is very close to 55.5 moles of water per liter of solution, or 55.5 M, one can substitute this number for the concentration of water and come up with the acidity constant, Ka, of the acid, as shown below. Acidity constants can often be measured fairly easily by tracking the neutralization of the acid with sodium hydroxide using a pH meter. If one considers the reverse reaction in the equilibrium shown below, it is obvious that the hydronium ion is reacting with the carboxylate anion to form the carboxylic acid and water. Almost as obvious, but not always noticed, is the fact that in this reverse reaction hydronium ion is functioning as Bronsted-Lowry acid (proton donor) and the carboxylate anion is functioning as a Bronsted-Lowry base (proton acceptor). The hydronium ion is called the conjugate acid of (the base) water. The carboxylate anion is called the conjugate base of the (the acid) acetic acid. In the same way, acetic acid is called the conjugate acid of the carboxylate (in this case acetate) anion and water is called the conjugate base of the hydronium ion. In any event, there is an acid and a base on each side of the equation, as there always is in an acid base reaction. Furthermore, the stronger acid and base are always on one side of the double arrows in the equation and the weaker acid and base are always on the other side. As astounding as that is, even more incredible is the fact that, at equilibrium, the weaker acid and weaker base will be present in higher concentration! What? How can the winners (stronger) lose out to the losers (weaker)? Think about what it means to be an acid or base and then what it would mean to be a stronger or weaker acid or base and you will see that this is the way it has to be. Experiment #10 Properties of Carboxylic Acids and Esters O O H 3C C O H + H 2O H 3C O O carboxylic (acetic) acid H 3C C O + H 3O C O carboxylate anion water H 3O H 3C = O H 3C Page 2 H 3O = O H 3C C O H H 2O C O hydronium ion C O H Keq 55.5M multiplying both sides of the above equation by 55.5M gives: O H 3C C O H 3O O H 3C C O H 55.5M = Keq x 55.5M = Ka 55.5M Ka (measured) for acetic acid = 1.76x10-5 Although water is not a strong enough base to convert substantial amounts of a typical carboxylic acid to the carboxylate anion a number of other bases are – among -1 -2 these are hydroxide, OH , carbonate, CO3 , and even the weakly basic bicarbonate, -1 HCO3 . Carboxylic acids containing up to four carbons are soluble in water. Beyond four carbons solubility decreases rapidly. This situation is similar to that of alcohols. When the carboxylic acid has a small hydrocarbon “tail” the ability of the carboxyl group to hydrogen bond with water causes the acid to be soluble. As the hydrophobic hydrocarbon tail grows in size, however, the acids become less soluble. As is the case with alcohols, the boiling points of carboxylic acids increase with molecular weight. As the boiling point increases the volatility decreases. Formic acid has a sharp odor and everyone knows what acetic acid (vinegar = 5% aqueous acetic acid) smells like. Acids having 3 or more carbons have unpleasant odors. Butanoic acid (CH3CH2CH2COOH) is redolent of rancid butter or Parmesan cheese. High molecular weight acids are odorless, presumably because they are not volatile enough to smell. When a carboxylic acid is reacted with an alcohol in the presence of a strong acid, which acts as a catalyst, an ester is formed along with a molecule of water. An example of this reaction is shown below where acetic acid reacts with isopentanol (3methylbutanol) to form the ester isopentyl acetate and water. Isopentyl acetate is sometimes called banana oil because it is mainly what gives bananas their fragrance and flavor. This type of reaction is known as the Fisher esterification, in honor of its discoverer Emil Fisher. It is an equilibrium reaction and the equilibrium constant is Experiment #10 Properties of Carboxylic Acids and Esters Page 3 typically about 1. This means that if one starts with one mole of carboxylic acid and one mole of alcohol, the result at equilibrium will be one-half mole of ester, one-half mole of water, one-half mole of carboxylic acid and one-half mole of alcohol. One way to improve the yield is to remove water from the reaction mixture as it is formed. This will drive the reaction in the forward direction and is an example of Le Châtelier’s principle. [Le Châtelier’s principle: If the conditions of a system, initially at equilibrium, are changed the equilibrium will shift in such a direction as to tend to restore the original conditions.] In this case the water is removed and by Le Châtelier’s principle the substances present will react to form more water. But by the chemical equation, if more water is formed more ester will be formed, also. Many esters, like isopentyl acetate, have pleasant fragrances and are used in perfumes and as flavorings. O CH3 CH3 C OH + HOCH2CH2CHCH3 Keq = C O CH2CH2CHCH3 C O CH2CH2CHCH3 + H2O H 2O ~ 1 CH3 O CH3 CH3 CH3 O CH3 CH3 O H3O+ C OH HOCH2CH2CHCH3 Since the Fisher esterification reaction is reversible it is possible to hydrolyze (from hydro, water, and lysis, to cut or break apart) an ester into a carboxylic acid and an alcohol. This reaction is also acid catalyzed. Esters can be broken apart under basic conditions, also. This reaction is called saponification because it can be used to make soap if one selects the appropriate ester (known as a triglyceride, usually obtained from animal fat) as starting material. The products of a saponification are an alcohol and the salt of a carboxylic acid as shown below. CH 3 O CH 3 C O CH 2CH 2CHCH 3 + Na OH CH 3 O CH 3 C O Na + HOCH 2CH 2CHCH 3 Objectives 1. To study some of the physical and chemical properties of carboxylic acids. 2. To prepare several esters and note their aromas. 3. To saponify two esters. Procedure Note: You may make the approximation that 20 drops of a liquid equals 1 milliliter. Experiment #10 Properties of Carboxylic Acids and Esters Page 4 Characteristics of acetic acid – Caution! Although acetic acid may smell like salad dressing, concentrated (glacial) acetic acid can cause severe burns. If any gets on you wash it off at once with lots of water. As usual, wear goggles. 1. Place 2 ml of 0.75M acetic acid into a test tube. Note and record the odor. Dip a glass stirring rod into the solution and touch the wet tip of the stirring rod to a piece of wide-range pHydrion paper. Compare the color of the paper with the chart that comes with the paper and record the pH of the solution. Repeat the above process with 0.75M samples that have been provided of 1-butanol and phenol. Record odors and pH values as above. 2. Add 2 ml of 1 M aqueous sodium hydroxide to each of the above solutions. Cork the test tubes and agitate. Remove the corks and determine the pH of each, as above. If not yet basic (pH > 7) add more sodium hydroxide by drops, agitating and testing pH as you go. When the solution becomes basic note any change in the odor and record the result. 3. Now add 2 M aqueous sulfuric acid dropwise to each tube, with agitation, until the contents of the test tubes are just acidic (test with pHydrion paper, pH <7; about 20 drops should be required). Note any change in the odors. Record the results. Clean the test tubes. Characteristics of Benzoic Acid – 1. Weigh 0.10 g of benzoic acid into a test tube. Add 2 ml of water. Agitate the test tube. Does the benzoic acid have an odor? How soluble is benzoic acid in water? O C OH 2. Add 1 ml of 2 M aqueous sodium hydroxide to the benzoic benzoic acid acid - water mixture. Stopper the tube with a cork and agitate the test tube in the usual way by holding the top of the tube with one hand and striking the bottom of the tube horizontally with the index finger of the other hand. Check with pH paper to be sure the mixture is basic (pH > 7); if not add more aqueous sodium hydroxide dropwise until the mixture is basic. Record any observations. 3. Measure and record the pH of the above mixture. Add 20 drops of 3 M hydrochloric acid to the above mixture, dropwise. After every second drop is added, agitate, check and record the pH, and note any changes. Saponification – 1. Place 20 drops of methyl salicylate and 10 ml of 6 M aqueous sodium hydroxide into a large test tube labeled “methyl salicylate saponification”. Record the odor of methyl salicylate. Place 10 drops of cooking oil and 10 ml of 6 M aqueous sodium hydroxide into a large test tube labeled “triglyceride saponification”. Place the tubes in a steam bath Experiment #10 Properties of Carboxylic Acids and Esters Page 5 and heat them for 30 minutes. Every 5 minutes, being careful not to burn yourself with the steam, remove the tubes from the steam bath and cork and shake them. After shaking, uncork them and return them to the steam bath. Note: To save time begin the “esterification” part of the experiment as the test tubes are steaming. 2. Cool the test tubes to room temperature using an ice-water bath. 3. Record the odor of the material in the methyl salicylate saponification tube. Then add 6 M hydrochloric acid to this tube, one milliliter at a time, until the solution is acidic. Stir with a glass rod after each addition and test with pHydrion paper. Record your observations as the solution becomes acidic. 4. Cork the tube labeled triglyceride saponification. Shake the tube and record observations. Then add 6 M hydrochloric acid to this tube, one milliliter at a time, followed by shaking. After each addition test with pHydrion paper. Record your observations as the solution becomes acidic. Fisher Esterification – Caution! Sulfuric acid is very caustic. If you get any on yourself flush the affected area immediately with lots of water. As usual, wear goggles. 1. Label five clean, dry test tubes with numbers corresponding to the compound pairs listed in the table to the right. Test Tube Carboxylic Acid Alcohol #1 formic isobutyl #2 acetic benzyl #3 acetic isopentyl #4 acetic ethyl #5 salicylic methyl 2. Add 10 drops of the corresponding carboxylic acid (0.1 g salicylic) to the numbered test tubes. Note and record the odor. Then add 10 drops of the indicated alcohol, recording the odor of the alcohol. 3. Add 5 drops of concentrated sulfuric acid to each test tube, stopper the tubes with corks and agitate. o 4. Loosen the corks in the necks of the tubes and place the tubes in a 60 C water bath for 15 minutes. Remove the tubes from the bath, allow to cool to room temperature, and add 2 ml of water to each. Remove a few drops of the top liquid layer from each test tube, in turn, placing them on a clean watch glass and note the odor. Can you pair the odors with the following: banana, raspberry, peach, wintergreen, and nail polish remover. [Many nail polish removers are mainly acetone, but some are based on one of these esters.] BK 8/03, Rev. 11/03