Week 10 - Esters
advertisement

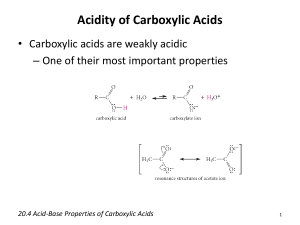
Survey of Chemistry I (CH 131) Page 1 of 8 Properties of Carboxylic Acids and Esters Background Carboxylic acids are structurally like aldehydes and ketones in that they contain the carbonyl group. However, an important difference is that carboxylic acids contain a hydroxyl group attached to the carbonyl carbon. —COOH The carboxylic acid group This combination gives the group its most important characteristic; it behaves as an acid. As a family, carboxylic acids are weak acids that ionize only slightly in water. As aqueous solutions, typical carboxylic acids ionize to the extent of only one percent or less. R—COOH + H2O → R—COO- + H3O+ At equilibrium, most of the acid is present as un-ionized molecules. Dissociation constants, Ka, of carboxylic acids, where R is an alkyl group, are 10-5 or less. Water solubility depends to a large extent on the size of the R-group. Only a few low-molecular-weight acids (up to four carbons) are very soluble in water. Although carboxylic acids are weak, they are capable of reacting with bases stronger than water. Thus while benzoic acid shows limited water solubility, it reacts with sodium hydroxide to form the soluble salt sodium benzoate. (Sodium benzoate is a preservative in soft drinks.) C6H5-COOH + NaOH → C6H5-COO-Na+ + H2O Benzoic acid Sodium benzoate Insoluble Soluble Sodium carbonate, Na2CO3, and sodium bicarbonate, NaHCO3 , solutions can neutralize carboxylic acids also. Survey of Chemistry I (CH 131) Page 2 of 8 The combination of a carboxylic acid and an alcohol gives an ester; water is eliminated. Ester formation is an equilibrium process, catalyzed by an acid catalyst. CH3CH2CH2 COOH + CH3CH2OH → H2O + CH3CH2CH2 COOCH2CH3 Butyric acid Ethyl alcohol Ethyl butyrate (Ester) Esterification → ←Hydrolysis The reaction typically gives 60% to 70% of the maximum yield. The reaction is a reversible process. An ester reacting with water, giving the carboxylic acid and alcohol, is called hydrolysis; it is acid catalyzed. The base-promoted decomposition of esters yields an alcohol and a salt of the carboxylic acid; this process is called saponification. Saponification means, “soap making,” and the sodium salt of a fatty acid (e.g., sodium stearate) is a soap. CH3CH2CH2COOCH2CH3 + NaOH → CH3CH2CH2COO-Na+ + CH3CH2OH Saponification → A distinctive difference between carboxylic acids and esters is in their characteristic odors. Carboxylic acids are noted for their sour, disagreeable odors. On the other hand, esters have sweet and pleasant odors often associated with fruits, and fruits smell the way they do because they contain esters. These compounds are used in the food industry as fragrances and flavoring agents. For example, the putrid odor of rancid butter is due to the presence of butyric acid, while the odor of pineapple is due to the presence of the ester, ethyl butyrate. Only those carboxylic acids of low molecular weight have odor at room temperature. Higher-molecular-weight carboxylic acids form strong hydrogen bonds, are solid, and have a low vapor pressure. Thus few molecules reach our noses. Esters, however, do not form hydrogen bonds among themselves; they are liquid at room temperature, even when the molecular weight is high. Thus they have high vapor pressure and many molecules can reach our noses, providing odor. Objectives: 1. To study the physical and chemical properties of carboxylic acids: solubility, acidity, and aroma. 2. To prepare a variety of esters and note their odors. 3. To demonstrate saponification. Survey of Chemistry I (CH 131) Page 3 of 8 Procedure Carboxylic Acids and Their Salts Characteristics of acetic acid 1. Place into a clean, dry small test tube (100 x13 mm) 2 mL of water and 10 drops of glacial acetic acid. Note its odor by wafting (moving your hand quickly over the open end of the test tube) the vapors toward your nose. Of what does it remind you? 2. Take a glass rod and dip it into the solution. Using wide-range indicator paper (pH 1—12), test the pH of the solution by touching the pH paper with the wet glass rod. Determine the value of the pH by comparing the color of the pH paper with the chart on the dispenser. 3. Now, add 2 mL of 2 M NaOH to the solution. Cork the test tube and sharply tap it with your finger. Remove the cork and determine the pH of the solution as before; if not basic, continue to add more base (dropwise) until the solution is basic. Note the odor and compare to the odor of the solution before the addition of base. 4. By dropwise addition of 3 M HCl, carefully reacidify the solution from step no. 3 (above); test the solution as before with pH paper until the solution tests acid. Does the original odor return? Characteristics of benzoic acid 1. You will weigh out 0.1 g of benzoic acid. Add the solid to a small test tube (100 x 13 mm) along with 2 mL of water. Is there any odor? Mix the solution by sharply tapping the test tube with your finger. How soluble is the benzoic acid? 2. Now add 1 mL of 2 M NaOH to the solution from step no. 1 (above), cork, and mix by sharply tapping the test tube with your finger. What happens to the solid benzoic acid? Is there any odor? 3. By drop-wise addition of 3M HCl, carefully reacidify the solution from step no. 2 (above); test as before with pH paper until acidic. As the solution becomes acidic, what do you observe? Esterification 1. Into five clean, dry small test tubes (100 x 13 mm), add 10 drops of liquid carboxylic acid or 0.1 g of solid carboxylic acid and 10 drops of alcohol according to the scheme in table Note the odor of each reactant. 2. Add 5 drops of concentrated H2SO4 to each test tube and mix the contents thoroughly by sharply tapping the test tube with your finger. Survey of Chemistry I (CH 131) Acids and Alcohols Test tube # Page 4 of 8 Carboxylic acid Alcohol 1 Formic Isobutyl 2 3 4 5 Acetic Acetic Acetic Salicylic Benzyl Isopentyl Ethyl Methyl 3. Place the test tubes in a warm water bath at 600C for 15 min. Remove the test tubes from the water bath, cool, and add 2 mL or water to each. Note that there is a layer on top of the water in each test tube. With a Pasteur pipet, take a few drops from this top layer and place on a watch glass. Note the odor. Match the ester from each test tube with one of the following odors: banana, peach, raspberry, nail polish remover, and wintergreen. Saponification This part of the experiment can be done while the esterification reactions are being heated. 1. Place into a large test tube (150 x 18 mm) 10 drops of methyl salicylate and 0.5 mL of 6 M NaOH. Heat the contents in a boiling water bath for 30 min. Record on the Data Sheet what has happened to the ester layer (1). 2. Cool the test tube to room temperature by placing it in an ice water bath. Determine the odor of the solution and record your observation on the Data Sheet (2). 3. Carefully add 6 M HC1 to the solution, 1 mL at a time, until the solution is acidic. After each addition, mix the contents and test the solution with pH paper. When the solution is acidic, what do you observe? What is the name of the compound formed? Answer these questions on the Data Sheet (3) Survey of Chemistry I (CH 131) Page 5 of 8 Esters Data Sheet NAME: ___________________________ DATE: ______________ PARTNER: ________________________ SECTION: ___________ Characteristics of Acetic Acid Property Water Solution NaOH Solution HCl Solution Odor Solubility pH Characteristics of Benzoic Acid Property Odor Solubility pH Water Solution NaOH Solution HCl Solution Survey of Chemistry I (CH 131) Page 6 of 8 Esterification Test Tube Acid Odor Alcohol 1 Formic Isobutyl 2 Acetic Benzyl 3 Acetic Isopentyl 4 Acetic Ethyl 5 Salicylic Methyl Odor Ester Odor Saponification 1.a What has happened to the ester layer when you added NaOH? 1.b Write the chemical equation for the saponification of methyl salicylate. 2. What has happened to the odor of the ester when cooled? 3. What forms on reacidification of the solution of methyl salicylate with HCl? Name that compound. Survey of Chemistry I (CH 131) Page 7 of 8 Esters Post-lab Questions NAME: ___________________________ DATE: ______________ SECTION: ___________ 1. How do carboxylic acids and esters differ in their characteristic odors? 2. Look in the Chapter Carboxilic acids and Esters in your textbook ( or consult library, Internet ) and find: A) Citric acid is present in _______ fruits, malic acid is present in __________, acetic acid is present in __________ and lactic acid is present in sour __________. Overuse of muscle tissue results in the buildup of __________ acid. The sting of an ant is caused by _________ acid. ___________acid is a constituent of butterfat. Spinach, cabbage and rhubarb contain __________ acid in addition to important vitamins. B) Which esters are responsible for the flavor and odor of: -raspberry -apricot -peach -pear -apple C) Aspirin, an ester of ______________ acid with ____________ acid, inhibits the synthesis of a class of hormones called ___________________, molecules that cause ________, ________ and ___________________ when present in the bloodstream in ____________________ levels. Survey of Chemistry I (CH 131) Page 8 of 8 Esters Pre-lab Assignment NAME: ___________________________ DATE: ______________ SECTION: ___________ 1. Write the structures of the following carboxylic acids: a. Acetic acid b. Formic acid c. Salicylic acid 2. Write the products from the reaction of benzoic acid and sodium hydroxide. 3. Octyl formate has the flavor of oranges. Name the alcohol and the carboxylic acid needed to synthesize this ester. 4. What is a soap?