Review with "youtube" Bohr-Rutherford Diagrams
advertisement

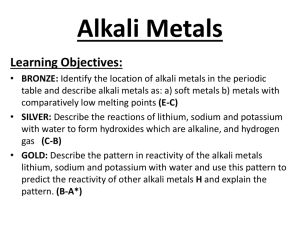
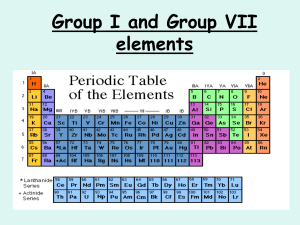
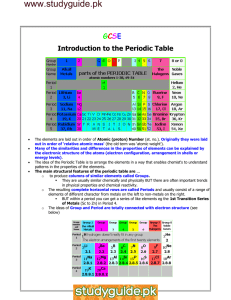
Review with "youtube" The Atom Song http://www.youtube.com/watch?v=vUzTQWn­wfE Bohr-Rutherford Diagrams Bohr­Rutherford diagrams are drawn to represent the electronic structure of elements. B­R diagrams have: ­ a circle to represent the nucleus ­ proton number and neutron number written in the nucleus ­ electron shells are a series of circles ­ electrons are black dots on circles ­ electrons are placed on an inside shell first ­ Rule is 2, 8, 8 for maximum electron placement 1 Practice: Draw a B­R diagram for Mg: Draw a B­R diagram for Cl: **check p. 92 for answers 2 An Alternate Representation "Human Bohr Model" http://www.youtube.com/watch?v=PLpZfJ4rGts Handout "Bohr-Rutherford Diagrams for the 1st 20 Elements 1. Complete B­R diagrams for elements #1 ­ 20 of the Periodic Table. 2. What trend do you notice about the electron arrangement of the alkali metals? alkaline earth metals? halogens? noble gases? 3. What trend do you notice about the electron arrangement of the first period? 2nd period? 3 Groups of the Periodic Table Using pp. 110 ­ 113 for help, answer the following questions: Noble Gases ­ Group 8: 1. Why are modern light bulbs usually filled with argon? 2. How does their outer electron arrangement help to explain their reactivity? The Alkali Metals ­ Group 1: 3. Describe the physical properties of alkali metals. 4. How does their electron structure explain their reactivity? 5. Explain the reaction of Na (sodium) and water? Why is that? The Halogens ­ Group 7: 6. Are they likely to react? 7. What happens to their electron configuration when they react? “A Group of One” 8. How is hydrogen like an alkali metal? 9. How is hydrogen unlike an alkali metal? Homework: Do Qu. 13. p. 101 and Qu. 7 p. 93. 4