UNIT 2 – LESSON 9: Heating Curve and Cooling
advertisement

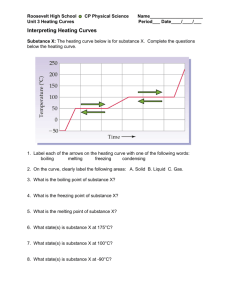
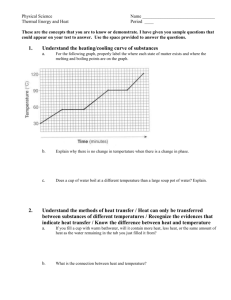
DEMOCRACY PREP FIRST AND LAST NAME 8th Grade Science SUBJECT DATE CLASS/HOMEROOM UNIT 2 – LESSON 9: Heating Curve and Cooling Curve Aim: IWBAT create and analyze heating and cooling curves. Do Now: Below is the heating curve for substance X. It starts as a solid below its melting point and is heated uniformly. 1. In which segments are phase changes occurring (circle all that apply)? a. AB b. BC c. CD d. DE e. EF 2. In which segments is kinetic energy changing (circle all that apply)? a. AB b. BC c. CD d. DE e. EF Name: ____________________________ Date ______________ States of Matter Quiz Question 1. Which of the states of matter has the greatest kinetic energy? a. Solid b. Liquid c. Gas 2. Which of the following states of matter generally has the greatest density? a. Solid b. Liquid c. Gas 3. What is the name of the process where a solid transitions directly to a gas? a. Evaporation b. Deposition c. Sublimation d. Fusion 4. All of the following events are generally occurring during melting except: a. The average kinetic energy of the particles remains constant b. The temperature is increasing c. The potential energy of the particles is increasing d. The distance between the particles is increasing e. The intermolecular forces of attraction are decreasing 5. Which process increases the potential energy of the particles in a sample? a. condensation b. deposition c. solidification d. vaporization Explanation Question 6. Looking at the heating curve on the right, what is the boiling point of this substance? 7. Looking at the heating curve on the right, what process is occurring between B and C? a. Solidification b. Melting c. Vaporization d. Condensation 8. Looking at the heating curve on the right, which segment represents an increase in potential energy, but not average kinetic energy. a. AB b. BC c. CD d. EF 9. Looking at the heating curve on the right, which segment represents where the entire sample is a liquid? a. AB b. BC c. CD d. EF 10. What happens to energy of a sample during a phase change from solid to liquid and why? a. Kinetic energy increases because molecules are moving faster, but potential energy stays the same. b. Kinetic energy decreases because molecules are moving slower, but potential energy stays the same. c. Potential energy increases because heat is being used to disrupt intermolecular forces, but kinetic energy stays the same. d. Potential energy increases because heat is being used to disrupt intermolecular forces, and kinetic energy increases because molecules are moving faster. Explanation Name: ________________________________ Date: __________________ Homework: Directions: Read the following information and answer the questions IN COMPLETE SENTENCES: Every pure substance has a melting point or a freezing point, a characteristic temperature at which it melts into a liquid or solidifies, and a boiling point or condensation point, a characteristic temperature at which it boils into a gas or condenses back to a liquid. The fact that pure substances have an individual melting point and boiling point can be used by chemists to identify substances. Freezing points of substances can be used to differentiate between ethanol (alcohol) and water, for example. Both of these are colorless liquids, but ethanol freezes at -­‐114.1°C, whereas water freezes at 0.0°C. This means that if you have a mixture of ethanol and water (such as beer or wine), the ethanol can be separated from water by cooling the mixture. The water will freeze first, and the liquid ethanol can be removed. Ethanol also boils at 78.5°C, while water boils at 100.0°C, so the two substances can also be separated by heating. The ethanol will boil first, and can be captured as a gas, while the liquid water is left behind. 1. What are the melting and boiling points of water in Celsius? 2. What are two ways you can separate ethanol from water? 3. Based on what you know about the following substances, would you say their melting point is higher or lower than water? Explain how you know for each one. a. Butter b. Salt c. Olive oil d. Copper e. Oxygen (O2)