3.1-Carbohydrates and Lipids
advertisement

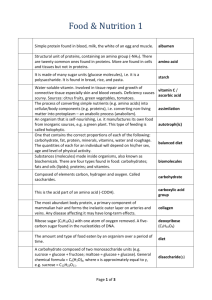
Nutrients Living things are made up of non-living chemicals These primarily include carbon (C), hydrogen (H), oxygen (O) and sometimes nitrogen (N) Macromolecules refer to proteins, carbohydrates, lipids (fats) and nucleic acids Nutrients refer to proteins, carbohydrates and lipids Carbohydrates Primary source of energy for the muscles and brain They are the body’s most important source of energy because they can be quickly broken down Make up largest part of most diets Only plants and some bacteria and protists can make their own carbohydrates via photosynthesis Carbohydrates Include: whole grain breads, rice, pasta, fruits & vegetables Bad sources (simple sugars): anything with refined sugar, ex. candy, soda, pastries, milk chocolate There are 3 main types of Carbohydrates: 1. Monosaccharides 2. Disaccharides 3. Polysaccharides 1. Monosaccharides Mono = one, saccharide = sugar Exists as a single sugar molecule Contain C, H, O in the 1:2:1 ratio Ex. CH2O Simple sugars which are the primary energy for all cells Ex. Glucose (C6H12O6) In all body cells; is used by mitochondria, to make energy 1. Monosaccharides Other examples: Fructose (sugar in fruit) ○ Sweeter than glucose Galactose (sugar in milk) Sugars end in “ose” Deoxyribose (sugar in DNA) 2. Disaccharides Di = two, saccharide = sugar Made up of 2 simple sugar molecules put together Made via “Dehydration Synthesis” Ex. Glucose + Glucose = Maltose (+ water) enzyme bond forms disaccharide ***A water (H2O) molecule comes off: hydroxide (OH) off one glucose and a hydrogen (H) off another 2. Disaccharides Glucose + Fructose = Sucrose (table sugar) Glucose + Galactose = Lactose (milk sugar) Comes from sugar canes, sugar beets, sugar maple trees 3. Polysaccharides Poly = many, saccharide = sugar Many sugar molecules linked in long, branching chains Some have 2000 - 6000 sugar molecules PLANTS: store excess sugar as starch, the sugar is broken down when needed by plant Cellulose: is a polysaccharide and a structural component of plant cell walls, it cannot be digested and used as energy but it is used as fibre 3. Polysaccharides ANIMALS: store energy as glycogen Glycogen: polysaccharide, similar structure to starch Chitin: polysaccharide that forms hard exoskeleton (external skeleton) in insects and crustaceans Lipids (fats) Secondary source of energy but are an excellent energy storage compound (better than carbohydrates) Contain C, H, O but different ratio 1:2:1 Difficult to be broken down Insulate the body, protect organs and makes up cell membranes Aid in vitamin absorption (fat-soluble vitamins A, E, K). 3 Types of Lipids Fats, oils, waxes Composed of 1 glycerol molecule and 3 fatty acids = triglyceride; formed by a dehydration synthesis reaction 1. 3 Types of Lipids Fats, oils, waxes Saturated: 1. single bonds between carbon atoms Come from animal fats and are more difficult to be broken down (solid fats) Ex. margarine, salad dressings, peanut butter, meats, FAST FOOD, milk products etc. ***Clogs arteries!!!*** 3 Types of Lipids Fats, oils, waxes Unsaturated: 1. double bonds between carbon atoms Come from plants and are easily broken down (liquid fats) Ex. fish fats, nuts, vegetable spreads, butter 3 Types of Lipids 3 Types of Lipids Phospholipids Similar to triglycerides but one fatty acid is replaced by a phosphate group (water soluble) 2. 3 Types of Lipids Phospholipids Polar end (hydrophilic-water loving) is soluble in water; non-polar (hydrophobic-water hating) ends are not soluble in water 2. 3 Types of Lipids Phospholipids Ex. SOAP: The hydrophobic tail traps the grease and the hydrophilic head (dissolves in water) washes it away 2. IMPORTANT structure of cell membranes!!! 3 Types of Lipids Steroids Large multiple-ring structure Include cholesterol, testosterone, estrogen and progesterone CHOLESTEROL: starting material for various hormones and is an important part of animal cell membranes 3. Can also combine with other fats to form blockages in arteries leads to heart disease and stroke Proteins Last resort for energy usage only used when body is in starvation mode Used to: build/repair cell structures (ex. muscles, hair) control rates of cellular reactions (=enzymes) Contain C, H, O, N and sometimes Sulphur (S) Made of “building blocks” called amino acids there are 20 different amino acids The type of protein made depends on the order and number of amino acids A closer look at amino acids It is made of an amino group, an acid group and an R group There are 20 different R groups, which give 20 different amino acids acid group amino group R group Proteins Each (of the 20) amino acids is attached to a transfer RNA (tRNA), that has a codon (3 base pairs...A,T,G,C) The codon has to match up to the base pairs on the messenger RNA (mRNA) Proteins Each (of the 20) amino acids is attached to a transfer RNA (tRNA), that has a codon (3 base pairs...A,T,G,C) The mRNA is read by a ribosome, a tRNA delivers an amino acid and the amino acid is added to the growing protein chain (called a polypeptide) Proteins Each (of the 20) amino acids is attached to a transfer RNA (tRNA), that has a codon (3 base pairs...A,T,G,C) Dipeptide: when two amino acids are formed Peptide Bond Amino acids are held together by a peptide bond, which is formed in a dehydration synthesis reaction These bonds are verystrong and difficult to break Denaturation The structure and function of a protein can be destroyed by heat (fever), radiation (UV rays) or pH (acidity) changes This changes the shape and folding of the protein A reversible change is called denaturation A permanent change is called coagulation ○ Ex. Egg in a frying pan The 3D shape of a protein is IMPORTANT Otherwise it will not function properly! What are classified as nutrients? Carbohydrates, lipids and proteins Which nutrient is the primary source of energy? Carbohydrates What reaction forms disacchrides and polysaccharides as well as proteins Dehydration synthesis **Draw a concept map of nutrients** Dehydration Synthesis & Hydrolysis 1. 2. Disaccharide Formation/Detachment Peptide Bond Formation/Detachment ***draw diagrams*** Activity and Homework Complete the NUTRIENTS worksheet It will be a homework check Read pages 30-42 Answer the following questions Page 36 #1, 3, 4 Page 39 #1-5 Page 42 #1-5 All questions will also be a homework check