Hydrolysis of Fats
advertisement

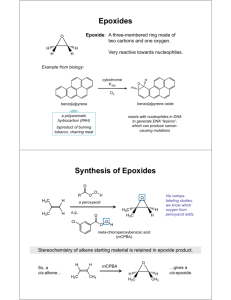
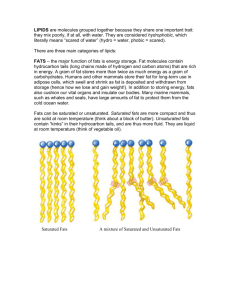
Hydrolysis of Fats Lesson 4 Hydrolysis of fats Oils and fats develop an unpleasant (rancid) smell if they are kept too long Rancid food has a bad smell, taste, texture/appearance Free fatty acids are generally absent in the fats of living animal tissue, but can form by enzyme action after the animal has died. Hydrolysis is when a compound splits when reacted with water. Fats are hydrolyzed in heat and water to their fatty acids. An example is shown Hydrolysis can happen quickly when certain microorganisms [bacteria, fungi, etc.] are present The reaction is catalyzed by the enzyme lipase [which helps the reaction move along faster without interfering in the reaction] Interesting Facts Fatty acids with 4 (butanoic), 6 (hexanoic) and 8 (octanoic) carbon atoms are released when the fats in milk and butter are hydrolysed Palmitic, stearic, and oleic acids are produced during the hydrolysis of chocolate and give it an oily/fatty flavour Hydrolysis also happens during deep fat frying(when water is produced from the food that is introduced to high temperatures) Oxidation Alkenes are more reactive than alkanes Similarly unsaturated fats are more reactive than saturated fats. The carbon-carbon double bond in unsaturated fats react with three things a. Oxygen (auto-oxidation) b. Hydrogen (hydrogenation) c. Light (photo-oxidation) Auto-Oxidation The oxidation of unsaturated fats by molecular oxygen, which occurs in air in the absence of enzymes, is called auto-oxidation When fat molecules break down to form unpleasant-tasting aldehydes and carboxylic acids, the process is known as oxidative rancidity Photo - Oxidation It is a free radical reaction which can be initiated by light Polyunsaturated oils contain a greater number of C=C double bonds and become rancid more quickly Oily fish (mackerel and herring) contain high percentages of unsaturated fatty acids and are prone to oxidation However oxidation, like hydrolysis, can be reduced by refrigeration Hydrogenation Hydrogenation can be added across the carbon-carbon double bond to decrease the level of unsaturation This is an important reaction as it increases the melting point, the hardness and chemical stability of the fat This reaction is used commercially to convert liquid oils into solid margarine Hydrogenation is carried out between 140 - 225 C in the presence of a finely divided metal catalyst (Zn, Cu, Ni) The degree of saturation can be controlled by varying the pressure of the hydrogen and the nature of the catalyst. Advantages of Hydrogenation Disadvantages of Hydrogenation Changes a liquid oil to a semi-solid or solid to make the melting point of an unsaturated fat more like that of a saturated fat Mono- and poly-unsaturated fats are healthier for the heart than saturated fats Decreases the rate of oxidation (stability increases with increasing saturation) In partial hydrogenation, trans fatty acids can form Increases Hardness Trans fatty acids are hard to metabolize, accumulate in fatty issues, are difficult to excrete from the body, increase levels of LDL (bad) cholesterol and are a low quality energy source Controls the feel and plasticity (stiffness)