Liquids and Solids
advertisement

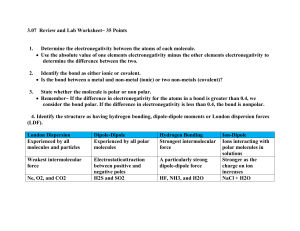
Liquids and Solids 1. Are liquids closer in physical properties to solids or gases? Why? Liquids are more similar to solids. There are many intermolecular forces experienced by solids and liquids and very few by gases. 2. Define a. Intramolecular Interactions Interactions (bonds) within a molecule. b. Intermolecular Interactions Interactions between two or more particles. 3. How do intermolecular Interactions affect the boiling and freezing point of a substance? Increased interactions results in a higher boiling point and higher freezing point. 4. Why is ∆Hvap much greater than ∆Hfus ? Because liquid gas transition breaks virtually all of the intermolecular bonds. 5. Types of Intramolecular Bonds and an example of each a. Non-polar (O2) b. Polar covalent (HF) c. Ionic (NaCl) d. Atomic ( Cgraphite ) 6. Types of Intermolecular Interactions a. London Dispersion (aka Van Der Waal’s forces or LDF) i. What type of substances experience this type of interaction? All polar and non-polar substances. ii. What occurs to make this interaction happen? Shift of e- to one side. iii. What affects the strength of this interaction? The size of the atom/molecule. increased mass = increased strength iv. What evidence suggests this type of interaction exists? The fact that non-polar substances can be condensed to liquids and solids. b. Dipole-Dipole Interactions i. What type of substances experience this type of interaction? Polar covalent substances. ii. What occurs to make this interaction happen? Dipoles line up due to attraction of partial charges. iii. What affects the strength of this interaction? The greater the polarity the greater the strength. c. Hydrogen bonding ( a special type of dipole-dipole interaction) i. What type of substances experience this type of interaction? Substances with polar N-H, O-H or H-F bonds. ii. What occurs to make this interaction happen? Dipoles line up due to attraction. This type of dipole is stronger than the typical dipole-dipole interaction because F, O and N are particularly electronegative. iii. What affects the strength of this interaction? The more polar the bond and greater the number of N-H, H-F or O-H bonds, the stronger the interaction. d. Ionic i. What type of substances experience this type of interaction? Ionically bonded substances. ii. What occurs to make this interaction happen? Positively and negatively charges line up. Na+Cl-Na+Cl-Na+Cl- etc…. 7. What is the typical ordering of strength for these interactions? Are there situations where this ordering may not apply? Ionic > Hydrogen Bond>Dipole-Dipole>London Dispersion There are occasions when this order may not apply. A very large non-polar substance compared to a mildly polar compound may have an LDF that is stronger than the dipole-dipole interaction. 8. Identify the most important types of interparticle forces present in the solids of each of the following: a. NH4Cl Ionic compund – ionic interactions b. Ar Atom – LDF only c. HF Polar – Hydrogen bonding and LDF d. BF3 Non-polar - LDF e. CHCl3 Polar – dipole-dipole and LDF 9. For which molecule in each of the following pairs would you expect the stronger intermolecular forces? a. CH3CH2CH2NH2 or H2NCH2CH2NH2 The answer has 2 areas of hydrogen bonding capability. b. CH3CH3 or H2CO The answer is polar so it has dipole-dipole interactions. c. CH3OH or H2CO Though both are polar, the answer is polar with the ability to hydrogen bond. d. HF or HBr Once again, both options are polar, but HF has the ability to hydrogen bond. 10. Characteristics of liquids a. Surface Tension – The resistance to increase surface area. i. How do the intermolecular interactions affect this characteristic? An increase in interactions leads to an increase in surface tension. b. Capillary Action – The spontaneous rising of a liquid in a narrow tube. i. What forces are responsible for this characteristic? 1. Adhesive – Interactions of molecule with the container. 2. Cohesive – Interactions between molecules with each other. ii. How do intermolecular interactions affect these forces? Increased interactions between molecules increases cohesiven interactions. Increased interactions between the molecule and container increases adhesive interactions. c. Viscosity – Resistance to flow. i. How do intermolecular forces affect this property? Increased interactions increased cohesive forces increased viscosity d. Vapor Pressure – Pressure above a liquid/solid due to evaporation. i. How do intermolecular forces affect this property? Increased interactions decreased vapor pressure ii. How does temperature affect this property? Increased temperature increased vapor pressure. 11. In each of the following groups of substances, pick the one that has the given property a. Highest boiling point: HBr, Kr, Cl2 The stronger the interactions the higher the boiling point. Kr and Cl2 are nonpolar – this means that they only experience LDFs. HBr is a polar molecule, which means that it experiences LDF and dipoledipole interactions. These forces are stronger. b. Highest freezing point: H2O, NaCl, HF The stronger the interactions the higher the freezing point. H2O and HF are both polar molecules that would experience LDF and hydrogen bonding intermolecular forces. NaCl, however, is an ionic compound. Thus it would experience, much stringer, ionic interactions. c. Lowest Vapor Pressure: Cl2, Br2, I2 The stronger the interactions the lower the vapor pressure. All of these compounds are nonpolar – meaning they experience only LDF interactions. Remember, the strength of the LDF very much depends on the size of the particle. Because I2 is the largest molecule, it would have the greatest LDF. d. Lowest freezing point: N2, CO, CO2 The weaker the interactions the lower the freezing point. CO is a polar molecule and CO2 and N2 are nonpolar. Nonpolar substances experience the weakest forces. Because N2 is smaller, it would experience the smallest LDF and, therefore, have the lowest freezing point. e. Greatest viscosity: H2S, HF, H2O2 The greater the intermolecular forces the greater the viscosity. H2S experiences dipole-dipole interactions and LDFs. Both HF and H2O2 experience H-bonding and LDFs. Because H2O2 has two areas for Hbonding it has the stringer intermolecular forces. f. Greatest heat of vaporization: CH3OCH3, CH3CH2OH, CH3CH2CH3 The greatest heat of vaporization would be associated with the compound that has the greatest intermolecular forces. CH3CH2OH is the only to experience H-bonding, thus it would have the highest heat of vaporization. g. Smallest enthalpy of fusion: I2, CsBr, CaO Both CsBr and CaO are ionic compounds and I2 is a non-polar compound. The enthalpy of fusion is amount of heat required to melt a substance (sl). The smallest enthalpy of fusion would be associated with the weakest intermolecular forces. Because I2 is non-polar it would only experience LDFs – the weakest intermolecular force and would therefore have the smallest ∆Hfusion. 12. Rationalize the difference in boiling point for: CH3CH2CH2CH2CH3 (36.2°C) (9.5°C) More surface area leads to more effective LDFs. Additionally, long molecules have the potential for tangling. 13. Which of the following substance would have a boiling point closest to argon? Cl2, HCL, F2, NaF, or HF Argon is non-polar and therefore has only LDF forces. That means we are looking for non-polar compounds from the list – only F2 and Cl2 fit the bill. Remember that LDFs are very much dependent upon the size/mass of the compound – so we need to determine whether Cl2 or F2 has a mass that is closer to Ar. The molar mass of Ar is 39 g/mol. F2 has a molar mass = 38 g/mol. These two would therefore have relatively similar LDFs and thus the closest boiling points. 14. Match the following boiling points with the correct structure; -42.1°C, -23°C and 78.5°C a. CH3CH2OH 78.5oC (strongest forces – H-bonding) b. CH3OCH3 -23oC (dipole-dipole interactions) c. CH3CH2CH3 -42.1oC (Weakest forces – LDFs) 15. Why does water create a concave meniscus and mercury a convex meniscus? Water’s adhesive forces are greater than its cohesive forces. Mercury’s cohesive forces are greater than its adhesive forces. 16. Why does water form into beads on a waxed car? Surface tension is used to minimize surface area. A bead, or sphere, is the shape with the lowest possible surface area. 17. What are two broad categories of solids? a. Amorphous (short range order, e.g. glass) b. Crystalline (long range order) 18. Types of crystalline solids a. Ionic b. Molecular c. Atomic d. Covalent Network e. Metallix 19. What is X-ray diffraction used for? X-ray diffraction is used to determine the structures of crystalline solids. 20. What is the Bragg equation? nλ = 2d sin θ n = integer (order) λ = wavelength used d = distance between atoms θ = angle of incidence/reflection This equation can be used to interpret the results of an diffraction experiment and determine the structure of a crystalline solid. 21. A topaz crystal has an interplanar spacing (d) of 1.36 x 10-10 m. Calculate the wavelength of the X-ray that should be used if θ = 15.0o (assume n = 1). These problems are typically just straight plug-ins. We know that we need to use the Bragg equation because we are dealing with X-ray diffraction. We will start by organizing our data: Plugging in: 22. What are 3 ways metals are typically arranged can be arranged? a. Face Centered Cubic (fcc) i. Illustrations ii. Facts 1. Volume = e3 r = radius of atom 2. # of nearest neighbors – 12 nearest neighbors 3 above, 3 below and 6 on the same level. 3. type of packing – Cubic Closest Packing 4. Total atoms within unit cell – 6(1/2) + 8(1/8) = 4 atoms 5. % of space used – 74% b. Body Centered Cubic i. Illustrations ii. Facts 1. Volume = e3 r = radius of atom 2. # of nearest neighbors – 8 nearest neighbors 4 above and 4 below 3. type of packing – You just need to know that it is not cubic closest packing 4. Total atoms within unit cell – 8(1/8) + 1 = 2 atoms 5. % of space used – 68% c. Simple Cubic (aka Primitive) i. Illustrations ii. Facts 1. Volume – e3 r = radius of atom 2. # of nearest neighbors – 6 nearest neighbors 1 above, 1 below and 4 on the same level. 3. type of packing - You just need to know that it is not cubic closest packing 4. Total atoms within unit cell – 8(1/8) = 1 atom 5. % of space used – 52.4% 23. A helpful formula for dealing with cubic structures and density: 24. A certain form of lead has a cubic closest packed structure with an edge length of 492 pm. Calculate the value of the atomic radius and density of the lead. Because this is a cubic closest packed structure (meaning face centered cubic): Plugging in: To solve for the density we can just plug into the formula from problem 23. Because we are talking about a face-centered arrangement, we know that there are four total atoms per unit cell. Plugging in: 25. You are given a small bar of an unknown metal X. You find the density of the metal to be 10.5 g/cm3. An X-ray diffraction experiment measures the edge of the face centered cubic unit cell as 4.09 x 10-10 m. Identify X. The identity of X can be determined by calculating the molar mass. We will do this once again by using the formula established in question 23. Once again, this is a face-centered cubic so we know that there are 4 total atoms per unit cell. 26. Theories for bonding in metals a. Electron Sea Model – cations regularly arranged e- move freely around. This theory explains metals ability to conduct because the electrons are described as free moving charged particles – the basic requirement for to conduct. b. Band Model – uses molecular orbitals to explain properties. In this theory we are looking at the distance separating filled molecular orbitals and the unoccupied molecular orbitals to explain the conducting/insulating properties of various solids. 27. Using Band Theory, explain the difference between conductors, semiconductors and insulators. In this model: A substance is described as a conductor when the filled MOs and unfilled MOs are in close proximity to one another. Because there is a very small energy difference, electrons are able to jump into the next unoccupied MO with relative ease. Thus the electrons are “free moving” charged particles. Semiconductors are substances who filled MOs and empty MOs have a greater separation than conductors but are close enough in proximity that electrons are still able to shift to the empty MOs. Because there is a greater separation it would take more energy (and thus be “harder”) to get the shift to occur, but it is still viable. Insulators are substances whose filled MOs and empty MOs have a significant difference in energy, and whose electrons, therefore, cannot move very freely between the two molecular orbitals 28. What are p-type and n-type semiconductors? P-type and N-type semiconductors are said to be “doped”. Meaning that they have has some impurity added to them to enhance their conducting abilities. Remember that the ability to conduct hinges on the movement of electrons. Silicon (Si) is a commonly used semi-conductor. If a silicon sample is taken and some of the silicon atoms are replaced with gallium a p-type semiconductor would be created. The “p” stands for positive. It has this namesake because while Si has 4 valence electrons, Ga only has 3 – thus there is one less electron leaving the sample “positive” by comparison. The reason that this enhances the conducting abilities is that with less electrons in the filled MOs there are “open” spaces created for remaining electrons to move into leading to a greater movement of charged particles (electrons) which leads to better conducting abilities. If, instead, a silicon sample is taken and some of the silicon atoms are replaced with arsenic, an n-type semi-conductor would be created. The “n” stands for negative. It has this naming because Aresenic has one additional valence electron compared to Si – thus there is one more electron making this sample more “negative” by comparison. The reason this enhances the conducting abilities is that with more electrons present, there are more charged particles to transition over the band separating the filled MOs and the unfilled MOs. More moving charged particles means more conductive ability. 29. What is a network atomic solid? What are examples? A solid that is made with covalent bonds throughout like Cdiamond 30. In molecular solids the intramolecular forces are very strong whereas the intermolecular forces are very weak. 31. The bonding in ionic solids will be primarily explained by the closest packing model. 32. What is typically larger, cation or anion? How does this affect the typical arrangement within a salt? Anions are typically larger due to e-/e- repulsion. Because of the consequences of sizing, typically the larger anion will be packed and the smaller cations will be in the holes (or spaces between packed anions). 33. What two types of holes in a closest packed structure? a. Tetrahedral b. Octahedral 34. Arrange these holes in order of increasing size. tetrahedral < octahedral 35. Guidelines for deciding which type of hole is used 0.225 R- < r+ <0.414 R0.414R- < r+ < 0.732 R- use tetrahedral use octahedral Where: “R – “ refers to the radius of the anion “r + “ refers to the radius of the cation 36. Why are holes that are slightly smaller than the cation used? Because when the cation is squeezed into the hold it pushes apart the anions a bit and that minimizes the anion/anion repulsion. 37. What is the ration of packed spheres to holes for a. Tetrahedral 1 : 2 (there are twice as many holes as spheres). b. Octahedral 1:1 (same number of spheres as holes) 38. Equation for vapor pressure change with temperature change 39. Label the following heating curve for water: 40. Label the following phase diagrams – how can you identify which is water? Water has a negative slope between S/L phases because ice is less dense than water. That means that if the pressure is increased (thereby forcing an increased density) water would convert to the liquid phase, as it is the most dense of all phases.