Section 2.3 Textbook Answers: pg. 82 #4-6, 8, 10
advertisement

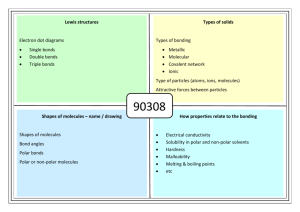
Section 2.3 Textbook Answers: pg. 82 #4-6, 8, 10-13, 16 4. Since this compound has a boiling point of -182C it is a gas at room temperature. 5. Compounds of polar molecules have stronger intermolecular bonds than non-polar molecules. A change of state such as melting requires the bonds between molecules (intermolecular bonds) to break. more energy is needed to break the stronger dipole-dipole bonds of polar molecules and they have a higher melting points than non-polar molecules. 6. Polar substances are soluble in polar solvents such as water (“like dissolves like”). Therefore, a compound that does not dissolve in water will be non-polar. Non-polar molecules tend to have lower melting points because it is easier to break the intermolecular bonds between the molecules (less attraction between the molecules) 8. If a compound has a very high melting and boiling point, it must have a very strong bond between the molecules (intermolecular bonds). In order for a molecule to melt or boil the intermolecular bonds must break and this requires energy. Ionic compounds have a very strong attraction due to the attraction of the positive and negative ions (electrostatic attraction). Ionic substances are polar are likely to be soluble in water which is also polar. 10. Water is polar it has a positive and negative side to the molecule. The positive ion of the ionic compound is attracted to the negative side of the water molecule. The negative ion of the ionic compound is attracted to the positive side of the water molecule. The ions of the salt (ionic compound) are pulled apart or dissociated and the substance dissolves. This is called an aqueous solution. 11. Since glycerol dissolves in water it must be a polar molecule. Since glycerol does not conduct electricity it cannot be ionic and must be a molecular (covalent) compound. Covalent compounds will not dissociate into ions in water and will not conduct electricity. 12. Ionic compounds can conduct an electric current when they are melted (liquids) or when they are dissolved in water (aqueous). In both of these cases the ions can move freely in solution. 13. No. Polar molecules do not dissociate into their ions when melted or when dissolved in water, therefore they cannot conduct electricity. 16. X is polar because it is soluble in water. Y is nonpolar because it is not soluble. Polar molecules have a higher melting points because of stronger intermolecular bonds.