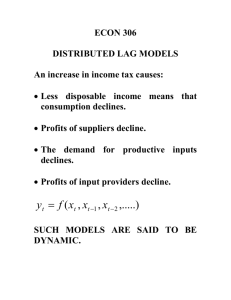
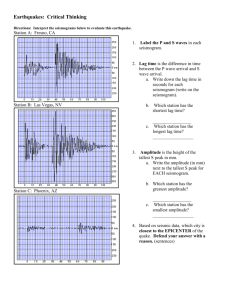
Source Monitoring: ERP Evidence for Greater Reactivity to
advertisement

36, 390–430 (1998) BR970979 BRAIN AND COGNITION ARTICLE NO. Source Monitoring: ERP Evidence for Greater Reactivity to Nontarget Information in Older Adults Jane Dywan, Sidney J. Segalowitz, and Lori Webster Brock University, St. Catharines, Ontario L2S 3A1, Canada Event-Related Potentials (ERPs) were collected concurrently with stimulus presentation during a source monitoring task. Younger adults were less likely than older adults to make source monitoring errors and their ERP records showed far greater discrimination between target stimuli and familiar but nontarget foils. Older adults not only made more source errors but produced high amplitude late positivities to the nontarget foils even when these foils were correctly rejected. Under divided attention conditions, younger adults performance was similar to that of the older adults both behaviorally and electrophysiologically. These data illustrate the role that attentional resources play in the ability to inhibit response tendencies and suggest that age differences in source monitoring may be more related to attentional control than inefficiencies in the encoding of contextual information. As well, they suggest that the ERP late positivity may represent a more general response to item salience rather than serve as an index of recollection as is the current view. 1998 Academic Press Key Words: aging; source memory; electrophysiology; ERPs; attention; inhibition; repetition effects; recognition. Older adults appear to have more difficulty than young adults when it comes to placing remembered events into the appropriate context with respect to time and place. Nonetheless, knowledge of source can be critical in the evaluation of ideas, in determining the appropriateness of the path one’s conversation is taking, or in making decisions regarding the quality of prodThis work was supported by grants to the authors from the Natural Science and Engineering Research Council of Canada. We thank Larry Jacoby, Janine Jennings, and Andy Yonelinas for discussions and advice on the lag paradigm used, Sharon Mercier and Sheila Lawson for their help in collecting these data, and Wendy Murphy and Tim Murphy for their organizational help. We also express our appreciation to the Medical Research Council Applied Psychology Unit, Cambridge, U.K., and Churchill College, Cambridge University, for providing support to us (J.D. and S.J.S.) as visiting scholars during the initial writing of this report. Address correspondence and reprint requests to Jane Dywan, Psychology Department, Brock University, St. Catharines, Ontario L2S 3A1, Canada. E-mail: jdywan@spartan.ac. brocku.ca. 390 0278-2626/98 $25.00 Copyright 1998 by Academic Press All rights of reproduction in any form reserved. ERPS AND SOURCE MONITORING 391 ucts or services being considered for purchase. It is, however, rare to be asked specifically about the contextual basis of a statement made or an opinion rendered. Source monitoring is typically a self-initiated process that must proceed online during active discourse or decision making. A breakdown in this online monitoring could lead to the kind of errors most readily attributable to growing egocentrism or declining judgement. BEHAVIORAL SOURCE MEMORY PARADIGMS An age-related dissociation between the ability to remember information and the ability to place that information in its appropriate context has been captured in the laboratory by a number of researchers. A common strategy involves presenting information from separate sources within the experimental context. As participants recall this information, they are asked to identify its source. Results are fairly consistent in documenting higher levels of source error among older adults. Older adults are more likely to confuse words rehearsed subvocally with words spoken aloud (Hashtroudi, Johnson, & Chrosniak, 1989), and to confuse imagined events with events that actually happened (Cohen & Faulkner, 1989; Hashtroudi, Johnson, & Chrosniak, 1990). The false fame paradigm is similar except that participants are not asked directly to make decisions about source. For example, in the famous names paradigm (Dywan & Jacoby, 1990), participants were asked to read a list of nonfamous names and were told that these names were nonfamous. They were later asked to make fame judgements on a new list of names, some of which were indeed famous and some of which were not. Included among the nonfamous names were some from the list that had been read earlier. Even though older adults were generally better than younger adults at distinguishing famous from nonfamous names, they were more likely than the younger adults to judge the previously presented nonfamous names as famous (see also Dywan, Segalowitz, & Williamson, 1994; Jennings & Jacoby, 1993; Multhaup, 1995). Bartlett, Strater, and Fulton (1991) demonstrated the same effect using famous and nonfamous faces. Source error effects are typically linked to an impairment in frontal lobe function and/or those cognitive processes associated with the frontal lobes (e.g., Craik, Morris, Morris, & Loewen, 1990; Dywan & Jacoby, 1990; Moscovitch, 1994; Schacter & Tulving, 1994; Shimamura, 1994). More specifically, Schacter and Tulving (1994) propose that source error in the context of adequate factual knowledge supports their formulation that episodic memory can be conceptualized as a memory system dissociable from semantic memory and dependent on prefrontal cortex. Of course, the frontal lobes have multiple roles in the processing of information (e.g., Diamond, 1990; Dywan & Segalowitz, 1996; Fuster, 1990; Godefroy & Rousseaux, 1996; Goldman-Rakic, 1988; Grafman, 1994; Malloy & Duffy, 1994; Petrides, 392 DYWAN, SEGALOWITZ, AND WEBSTER 1994) so that making this association on its own does not markedly improve our understanding of the specific processes involved in the breakdown of source memory. Some attempts to account for source error focus primarily on factors affecting the encoding of relevant information. It has been suggested that source error occurs because of a failure to notice, integrate, or retain specific details associated with the initial event (Hashtroudi et al., 1989, 1990; Shimamura and Squire, 1987). There is certainly evidence indicating that older adults are less likely than younger adults to recollect the modality in which successfully recalled words were originally presented (e.g., Lehman & Mellinger, 1984; Light, La Voie, Valencia-Laver, Albertson-Owens, & Mead, 1992), they are less likely to remember the order of recalled events (Kausler & Wiley, 1990), the spatial location of otherwise successfully recalled pictures (Zelinski & Light, 1988), or the spatial location of objects that had been recalled (Puglisi, Park, Smith, & Hill, 1985). Other formulations give more weight to the strategic aspects of retrieval. For example, we (Dywan et al., 1994) have proposed that aging leads to a reduced ability to ignore nonrelevant aspects of ongoing experience or to inhibit response tendencies that are not compatible with task goals. From this perspective, source error in the famous names (or faces, e.g., Bartlett et al., 1991) paradigm could be seen as a failure to inhibit one’s response to the familiarity of recently repeated events. In this case, the prepotent response tendency would be to interpret familiarity as fame. More generally, in situations where the potential for confusion is high, the individual would have to inhibit a salient response, i.e., based perhaps on perceptual fluency, in order for appropriate metacognitive judgments to occur (see also, Diamond, 1990; Jennings & Jacoby, 1993; Masson, Carroll, & Micco, 1995; Moscovitch, 1994; Shimamura, Jurica, Mangels, Gershberg, & Knight, 1995). The inhibitory regulation hypothesis (Hasher & Zacks, 1988) provides a similar formulation except that the role of inhibitory control as a gate to working memory is emphasized. Finally, Johnson and Chalfonte (1994) propose that self-generated reactivation of encoded information serves to bind together and strengthen aspects of memory and that binding deficits contribute to source memory impairments. However, the broader source monitoring framework (Johnson, Hashtroudi, & Lindsay, 1993) allows for the possibility that aging might affect both the encoding and retention of details as well as the more evaluative/ strategic processes including criteria such as what information will be considered in making source memory judgements. All these explanations seem compatible with the nature of the phenomenon but there is little direct evidence to support one over the others. Psychometric indices of frontal function have been shown to relate to source error (Craik et al., 1990) but these relationships have been difficult to replicate (e.g., Johnson, De Leonardis, Hashtroudi, & Ferguson, 1995; Spencer & Raz, 1994). In an earlier study using the famous name paradigm (Dywan et al., ERPS AND SOURCE MONITORING 393 1994), we examined source monitoring as it related to the tendency to make perseverative errors on the Wisconsin Card Sorting Test (WCST, Heaton, 1981) and to participants’ general response tendencies as measured electrophysiologically using the Contingent Negative Variation (CNV), a frontally generated event-related potential (ERP) (Rosahl & Knight, 1995). We found no relationship between the WCST and source error. However, both WCST perseverative errors and source error were related to the CNV but to different parts of the waveform. WCST errors were related to the initial orienting portion of the wave, whereas source errors were related to the later portions of the wave, that part of the wave thought to reflect the anticipation of a response. This was consistent with our formulation that source error may involve a failure in response inhibition. However, this inference is still indirect in that the CNV was gathered during a simple tone monitoring task and could serve only as a general index of a subject’s response tendencies. It was not an ERP correlate of ongoing cognitive processes during the source monitoring task itself. EVENT RELATED POTENTIAL (ERP) PARADIGMS Because of their ability to reveal momentary changes in the pattern of brain activation, the technique of recording ERPs is ideally suited to provide insights into the processes that accompany ongoing cognitive operations (Johnson, 1995; Pribram & McGuinness, 1992). Measuring ERPs while younger and older adults are engaged in source monitoring might therefore reflect the neural responsivity that accounts for the frequently observed difference in their performance. It is well established that ERPs are sensitive to the previous occurrence of information (e.g., Bentin & McCarthy, 1994; Fabiani, Karis, & Donchin, 1986; Friedman, 1990; Paller & Kutas, 1992; Rugg, 1985; Smith, 1993). Relative to the first presentation of an item, the second presentation elicits a more positive amplitude in the later portion of the waveform, sometimes referred to as a late positive component. This ERP ‘‘repetition effect’’ is observed in the performance of older adults to about the same degree as it is in younger adults (Hamberger & Friedman, 1992; Friedman, Berman, & Hamberger, 1993; Friedman, Hamberger, Stern, & Marder, 1992; Rugg, Pearl, Walker, Roberts, & Holdstock, 1994). Interestingly, even Alzheimer patients have been shown to produce an ERP repetition effect that is not significantly different in amplitude from those produced by healthy agematched controls (Friedman et al., 1992; Rugg et al., 1994). Karayanidis, Andrews, Ward, and McConaghy (1993) reported that older adults produced repetition effects of longer duration than younger adults, and Friedman, Hamberger, and Ritter (1993b) found that older adults produced repetition effects that were of both greater amplitude and longer duration than their younger counterparts. Thus, the recognition effect takes the same form in older and younger adults and in some cases appears slightly more robust in older subjects. 394 DYWAN, SEGALOWITZ, AND WEBSTER The similarity in repetition effects across age groups may reflect the relatively passive mode of response required of subjects in the typical ERP repetition paradigm. First, in order to optimize signal-to-noise ratios in ERP studies, researchers use large sets of items in each response category, e.g., Hamberger and Friedman (1992) used 8 blocks of items with 36 new items and 36 repeating items for a total of 108 items per block. Karayanidis et al. (1993) presented participants with two stimulus lists of 200 items each; Rugg et al. (1994) used a list of 421 words, 80 of which were presented twice after a lag of one intervening item and 80 of which were presented twice after a lag of 6 intervening items. Thus, the likelihood of specific recollection of individual items would be low. Individuals would be forced to rely on a differential sense of familiarity that would attend previously presented relative to new items. Second, the repetition effect is often monitored indirectly, in that participants are asked to make unrelated category judgements (e.g., Hamberger & Friedman, 1992; Rugg et al., 1994) or lexical decisions (e.g., Karayanidis et al., 1993). In neither case are participants required to engage in active recollection of the repeated words. Source monitoring paradigms may provide a more sensitive test of age differences in recognition memory. The highly consistent age-related effects observed in source monitoring studies reflect the difference, not between old versus new items, but between items or events both of which are ‘‘old’’ or familiar. Familiarity, in this case, is associated with different contexts. Accuracy would depend on the ability to respond to familiarity linked with one context but to withhold a response to familiarity linked with some other context either within or outside of the experimental situation. Thus, source monitoring judgements, unlike simple recognition judgments, are more likely to elicit active information processing strategies. Examining ERP responses while younger and older adults are in the process of monitoring the source of item familiarity may reveal neurophysiological response patterns associated with the higher source error rates typically observed in older adults. STUDY 1 In the first study, we examined the ERP repetition effect in older and younger adults within the framework of a source monitoring paradigm. We hoped to determine whether age-related changes in the ability to discriminate between sources of familiarity would be reflected in a differential ERP amplitude for two types of recently seen items. Both types of item should be experienced as familiar within the experimental context, but only one type should be considered a target with respect to the source monitoring task. In this case, a response based solely on a passive experience of familiarity would lead to source error. We take our paradigm from one developed by Larry Jacoby and his colleagues, e.g., Jennings and Jacoby (1997), whereby participants are given a list to study and are subsequently asked to do a running ERPS AND SOURCE MONITORING 395 recognition task in which the study words are interspersed with novel foils. Some of the foils repeat after a lag of six words. Participants are told about the repeating foils but asked to ignore them. Their task is simply to indicate when they detect words from the previously read study list. Jennings and Jacoby have demonstrated that older adults are more likely to misclassify repeated foils as study words after a lag of as little as three items. The ability to make correct source judgments cannot depend on the experience of familiarity alone. Even though the experience of familiarity can be a good index of previous occurrence, this experience on its own may not necessarily provide information about the context in which the familiarity was acquired. The question, therefore, is how the experience of familiarity is modulated in order to prevent source error from occurring and how this modulation may change across the lifespan such that source error becomes a more likely occurrence. If younger and older adults experience the familiarity of previous events to about the same degree, then it may be that younger adults engage in an extra evaluation to establish the context of the event. Source monitoring would, therefore, constitute a secondary process that can be activated when an individual is faced with a difficult mnemonic discrimination. Older adults may be either less able to engage this metacognitive process or they may be less likely to appreciate that the extra monitoring is required. Another possibility is that younger adults experience a more differentiated memory than older adults (i.e., one with more potentially source-specifying information). If this were the case, it would not be necessary to engage in an extra evaluation in order to make a correct source attribution, one could rely on automatic response tendencies without much risk of source error. We were hoping that the ERPs gathered as subjects made decisions about the source of item familiarity would reflect those processes that underlie the age effects so consistently demonstrated in behavioral response. Method Participants Participants were 12 active older adults from the local community (M age ⫽ 69.8 years) and 11 younger adults (M age ⫽ 23.9 years) who volunteered to take part in a study on age and cognition. The groups did not differ with respect to years of education (M ⫽ 13.75, SD ⫽ 2.34, for the older adults and M ⫽ 14.09, SD ⫽ 1.64, for the younger adults), t(21) ⫽ .40, ns. An initial screening eliminated participants with a history of substance abuse, cerebrovascular or cardiovascular accident, psychiatric disorder, traumatic brain injury, nonnormative levels of cognitive decline, or health problems that would interfere with normal performance. Community volunteers were paid a nominal fee to cover incidental expenses, if any, and those young adults who were undergraduates received course credit for their participation. 396 DYWAN, SEGALOWITZ, AND WEBSTER Stimuli The stimulus set consisted of 100 five-letter words, all presented in upper case. The 100 words were divided into four lists balanced for frequency of occurrence according to the Kučera and Francis (1967) norms (overall mean frequency ⫽ 292 per million, F(3, 96) ⫽ .03, ns). Four formats were developed, such that one list was used as study items, another as lag items, and the other two as foils. The four lists were rotated so that each list appeared in each category, as study items, as lag items, and twice as foils. The formats were run sequentially as subjects were tested so that differences in ERP results could not be attributed to characteristics of a particular word list or order of presentation. ERP memory studies are typically based on large numbers of items in each list to ensure adequate signal to noise ratios when averaging the ERP waveforms. However, our goal was to pit recollection against familiarity which in other studies are usually confounded. Presenting participants with a study list of more than 25 words would represent a considerable departure from the behavioural source memory paradigm. The larger the target list, the more likely participants, particularly the older participants, would adopt a passive, familiarity-based response mode and this, we felt, would jeopardize our chances of seeing active and passive response tendencies in competition. Procedure Participants were presented with a list of 25 study words, each presented for 5 seconds on a computer screen. After a 2-minute rest they were presented with a test list of 125 words, each word remaining on the screen for 1 second or until the participant responded by a key press (‘‘yes’’ or ‘‘no’’). The test list consisted of the 25 study words, 25 new words that would be repeated after a lag of six interposed items, and 50 new words that were not repeated. Participants were informed that some of the new test words would repeat but that these should not be mistaken for study words. It was stressed that they should hit the ‘‘yes’’ button only for those words that had been read in the previous list and the ‘‘no’’ button for all others. Word type (study, lag, foil) in the test lists were pseudo-randomized to prevent selective fatigue effects. ERPs were 1100 ms epochs (with 100 ms prestimulus baseline) timelocked to the presentation of the words. They were recorded from midline prefrontal (FPz), frontal (Fz), central (Cz), and parietal (Pz) sites (referenced to linked ears), with a bilateral eye electrode for monitoring eye movements and a mastoid ground. Only results from Fz, Cz, and Pz are presented.1 Im1 Results at the FPz site were similar to those at the other sites but less stable. The FPz site was useful, however, in helping to detect eye movement artifact. 397 ERPS AND SOURCE MONITORING TABLE 1 Proportion of Words Judged to Be from the Study List (pStudy) and Latency of Behavioral Response (RT) by Type of Word for Younger and Older Adults (Study 1) Younger adults Type of word Study M SD Lag M SD Foils M SD Older adults pStudy RT (ms) pStudy RT (ms) 0.58 0.09 799 126 0.59 0.17 1011 146 0.17 0.13 821 149 0.40 0.19 1171 253 0.09 0.08 748 130 0.11 0.07 1044 193 Note. Response times are for correct decisions only. pedances were kept below 5 kOhms; all signals were amplified with a gain of 40,000 and were digitized at 400 Hz with a 12-bit A–D window. Bandpass was .5–30 Hz. ERPs were averaged only from those trials to which the participant made a correct response. The automatic artifact rejection window was set at ⫾100 µV and electrooculographic activity (EOG) was corrected on each trial using linear regression procedures (e.g., Verleger, Gasser, & Mocks, 1982). Subjects with too few artifact-free trials to produce a stable ERP were eliminated reducing the size of the older adult group to 10 for ERP analyses. Huynh–Feldt corrections for multiple comparisons were used when appropriate; the corrected p values and uncorrected degrees of freedom are reported. Results Behavioral Data Table 1 indicates the percentage of items designated as a study word for each of the stimulus types. These data were subjected to a 2 ⫻ 3 repeated measures analysis of variance (ANOVA) with group (older vs younger adults) as the between-group factor and stimulus type (study word vs lag word vs foil) as the within-group factor. Results indicated a main effect for group, F(1, 21) ⫽ 5.56, p ⫽ .03, η 2 ⫽ .21, with the older adults (M ⫽ .37) more likely to designate items as targets than the younger adults (M ⫽ .28).2 There was a main effect for stimulus type, F(1, 42) ⫽ 106.0, p ⬍ .001, η 2 ⫽ .83, such that the probability of correctly responding ‘‘yes’’ to the 2 Strength of association is indicated by η 2. The denominator in each case contains only variance attributable to the effect of interest plus error. 398 DYWAN, SEGALOWITZ, AND WEBSTER study words (M ⫽ .60) was greater than the probability of responding ‘‘yes’’ to the lag words (M ⫽ .29) and the probability of responding ‘‘yes’’ to the lag words was greater than responding ‘‘yes’’ to the foils (M ⫽ .10). There was also a group by stimulus type interaction, F(2, 42) ⫽ 6.28, p ⬍ .025, η 2 ⫽ .23, such that the tendency to say ‘‘yes’’ to study items was very similar between older (M ⫽ .59) and younger (M ⫽ .58) adults, as was the tendency to say ‘‘yes’’ to foils (M ⫽ .11 vs M ⫽ .09 for older and younger adults, respectively). However, the older adults were more than twice as likely to designate lag words as study items (M ⫽ .40) as were the younger adults (M ⫽ .17). These results replicate the frequently demonstrated tendency of older adults to make incorrect attributions about the source of item familiarity. Reaction Times Reaction times (RTs) to correct acceptance of study items and correct rejection of lag and foil items (Table 1) were submitted to a 2 ⫻ 3 group (older vs younger adults) by stimulus type (study correct vs lag correct vs foil correct) ANOVA. In general, behavioral responses were slower for the older adults (M ⫽ 1075 ms) than for the younger adults (M ⫽ 789 ms), F(1, 21) ⫽ 17.79, p ⬍ .001, η 2 ⫽ .46. There was also a main effect for type of item, F(2, 42) ⫽ 12.27, p ⬍ .001, η 2 ⫽ .37, indicating that correct decisions regarding lag items involved longer RTs (M ⫽ 1003 ms) than correct decisions about study items (M ⫽ 909 ms) or foils (M ⫽ 902 ms). RTs for study and foil items were not reliably different. There was also a group X stimulus type interaction, F(2, 42) ⫽ 4.94, p ⬍ .02, η 2 ⫽ .19, such that younger adults were faster making a correct response to foil items (M ⫽ 748 ms) relative to study items (M ⫽ 799 ms) and to lag items (M ⫽ 821 ms) and the older adults were faster at the study items (M ⫽ 1011 ms) and foil items (M ⫽ 1044 ms) relative to the lag items (M ⫽ 1171 ms). Thus, while lag items generally required more time than study items, older adults were slowed down more (14%) than were the young (6%). ERP Data The repetition effect. ERPs measured at midline sites, Fz, Cz, and Pz were averaged separately across repeated and nonrepeated words (see Fig. 1). ERPs for the ‘‘old’’ items were averaged over all correctly classified words that subjects had seen earlier in the experimental context (study words and lag words), whereas the ERPs for ‘‘new’’ items were averaged across correctly classified words that had been presented for the first time during recognition (foil words and the first presentation of lag words). We first calculated area under the curve at 50-ms intervals relative to the 100-ms prestimulus baseline for each stimulus type. Paired t tests of areas at each 50-ms interval for old relative to new items (Fig. 2) indicate that in the younger group differentiation between previously seen and new items ERPS AND SOURCE MONITORING 399 FIG. 1. Grand average ERPs generated by younger and older adults to words previously seen (old) and words not previously seen (new) irrespective of context (Study 1). Fz, Cz, and Pz signify midline frontal, central, and parietal sites. Duration of the ERP extends from ⫺100 (prestimulus) to 800 ms poststimulus. began between 300 and 400 ms and was maintained until about 600 ms at all sites. The familiar/unfamiliar distinction was observed in the older adults’ data as well, beginning between 200 and 300 ms and was maintained until about 800 ms, 200 ms longer than in the younger group. Shaded areas in Fig. 2 represent those intervals in which the difference in ERP amplitude between old items and new items had p values less than .10. The range of p values represented by the shaded areas is from p ⫽ .097 to p ⫽ .002. Given the well documented speed of processing differences between older and younger adults, it was important to ensure that the late positivities of both groups were represented within the areas being compared. This is a conservative approach. When we take a components approach, i.e., when we compare the area of maximum differentiation for each group, effect sizes are much larger than they are when we use the same time frame for all groups but the component approach has its own problems. It requires that 400 DYWAN, SEGALOWITZ, AND WEBSTER FIG. 2. ERP amplitude in microvolts (µV) at 50-ms intervals relative to 100-ms prestimulus baseline as elicited by previously seen words (old) and words not previously seen (new) irrespective of context for younger and older adults (Study 1). Each data point represents 50 ms leading up to the figure on the X axis. Shaded areas represent intervals in which the difference in amplitude between old and new items had p values of less than .10 (range: p ⫽ .097 to .002). one compare peaks emerging at different time frames within the same ANOVA and there are those who would feel uncomfortable with this practice. To compare groups with respect to the familiar/unfamiliar distinction, the areas from 350 to 800 ms were combined to represent a late positivity (mean amplitude per sampling point). This time frame encompassed areas of significant differentiation across sites for both younger and older participants in this study and was within the area found to be sensitive to recognition effects in previous studies (e.g., Friedman et al., 1993b). These late positivities were entered into a 2 ⫻ 2 ⫻ 3 ANOVA comparing group (younger adults vs older adults), stimulus types (‘‘previously seen’’ vs ‘‘not previously seen’’), and site (Fz, Cz, and Pz). Results indicated that, in general, older ERPS AND SOURCE MONITORING 401 adults produced ERPs of greater positivity (M ⫽ 2.37 µV) than did younger adults (M ⫽ .75 µV) but this difference did not reach statistical significance, F(1, 19) ⫽ 3.26, p ⫽ .09. There was an effect for stimulus type such that previously seen items elicited greater amplitudes (M ⫽ 2.11 µV) than new items (M ⫽ .92 µV), F(1, 19) ⫽ 8.55, p ⬍ .01, η 2 ⫽ .31. The positivity as measured at the Fz electrode placement was greater (M ⫽ 2.08 µV) than at the other two sites, Cz (M ⫽ 1.18 µV) and Pz (M ⫽ 1.29 µV), respectively, but this difference was not reliable, F(1, 19) ⫽ 3.12, ns. and there were no interactions between group, site, and stimulus type.3 These analyses indicate that the old/new effect was evident in both groups and that frontal sites produced a marginally greater positivity than more posterior sites but these differences were not related to the old/new discrimination the participants were making. As well, there was no indication that the old vs new repetition effect was reliably greater for either group except that it persisted somewhat longer among the older adults. The source monitoring effect. The next set of analyses were done to determine the degree to which the ERP late positivities of younger and older adults were sensitive to the distinction between two types of familiar items, both of which had been repeated but in different contexts. In this case, the comparison of interest was between ERP amplitudes elicited in response to study words, which were considered targets, and nontarget lag words, which were familiar because they had just been repeated during the recognition task. ERPs to foils, new words presented only once during test, were included in all ANOVAs to control for general levels of reactivity. Averaged ERPs for study words, lag words, and foils elicited during the test phase of the experiment are presented in Fig. 3 for younger and older adults. As in the previous analysis, we first calculated area under the curve at 50-ms intervals relative to the 100-ms prestimulus baseline for the comparison of interest, in this case, between study words and lag words (Fig. 4). Paired t tests of areas at each 50-ms interval indicate that for the younger adults the greatest differentiation between study and lag items occurred between 400 and 600 ms and that the amplitude of the late positivity is greater for study items relative to lag items. However, in the case of the older adults the pattern is reversed. The greater amplitude is elicited by the lag words rather than by the study words. This appears relatively consistent across the intervals but is most reliable from 250 to 600 ms. The shaded areas in Fig. 4 represent those intervals in which the difference in ERP amplitude between study items and lag items had p values less than .1 (the range is p ⫽ .090 to .001). 3 A normalizing procedure (e.g., McCarthy & Wood, 1985) is recommended in order to ensure that site by condition interactions are not due simply to the disproportionate range in amplitudes in anterior relative to posterior electrode sites. In this study, there were no interactions between conditions and site using the full range of amplitudes so that normalizing amplitudes across sites was not necessary. 402 DYWAN, SEGALOWITZ, AND WEBSTER FIG. 3. Grand average ERPs generated by younger and older adults to study words, lag words, and foils (Study 1). To compare groups, the areas from 250 to 600 ms were combined in order to encompass the areas of maximal differentiation for both groups.4 These late positivities were entered into a 2 ⫻ 3 ⫻ 3 ANOVA comparing groups (younger adults vs older adults), stimulus type (study words vs lag words vs foils), and sites (Fz, Cz, and Pz). There was no main effect of group, F(1, 19) ⫽ 0.36, ns. There was, however, a main effect for stimulus type, F(2, 38) ⫽ 6.08, p ⬍ .01, η 2 ⫽ .24. ERP amplitude to foils was generally less positive (M ⫽ .97) than for either lag words (M ⫽ 2.17) or study words (M ⫽ 2.64). There was a main effect for site, F(2, 38) ⫽ 5.09, p ⫽ .01, 4 Although lag items produced some ERP elevation prior to 250 ms at the Fz site for older adults, the only differences to exceed the probability level of .05 at Fz were for the area between 250 and 300 ms (t(9) ⫽ 2.30, p ⫽ .047) and again between 450 and 500 (t(9) ⫽ 2.54, p ⫽ .031). As well, this early difference did not approach significance at the Cz and Pz sites. Thus, the area from 250 to 600 ms was considered the most reliable for both groups. ERPS AND SOURCE MONITORING 403 FIG. 4. ERP amplitude at 50-ms intervals relative to 100-ms prestimulus baseline as elicited by lag words and study words for younger and older adults (Study 1). Shaded areas represent intervals in which the difference in amplitude between study words and lag words had p values of less than .1 (range: p ⫽ .090 to .001). η 2 ⫽ .21, with higher amplitude at Fz (M ⫽ 2.41 µV) than at Cz (M ⫽ 1.38 µV) or Pz (M ⫽ 1.93 µV). There was also a group by site interaction, F(2, 38) ⫽ 6.54, p ⬍ .01, η 2 ⫽ .26, indicating a tendency for increased frontality in older adults. The amplitude of the late positivity for older adults was greatest at Fz (M ⫽ 3.13 µV) and gradually reduced in size at more posterior sites, Cz (M ⫽ 1.95 µV), and Pz (M ⫽ 1.51 µV). The amplitude of the late positivity for younger adults was greatest at the most posterior site, Pz (M ⫽ 2.32 µV), Cz (M ⫽ 0.88 µV), Fz (M ⫽ 1.77 µV). What is of most interest, however, was a significant group by stimulus-type interaction, F(2, 38) ⫽ 6.24, p ⫽ .005, η 2 ⫽ .25, confirming that the older adults produced a greater positivity across sites to the lag words (M ⫽ 3.14 µV) relative to study words (M ⫽ 1.94 µV) or foils (M ⫽ 1.51 µV), while the younger 404 DYWAN, SEGALOWITZ, AND WEBSTER adults produced the greatest positivity to study words (M ⫽ 3.34 µV) relative to lag words (M ⫽ 1.19 µV) or foils (M ⫽ .43 µV). Group effects for stimulus type did not interact with site (see footnote 3). Summary and Discussion As expected, older adults were more likely than younger adults to make source errors. There was no difference in the response of older and younger adults to study words and foils but the older adults were twice as likely as the younger adults to make source errors when new words were presented for a second time during the recognition test. While predicted, these behavioral data do not advance our understanding of those preresponse factors that lead to the higher rates of source error in older adults. It was hoped that ERPs collected online while participants made source monitoring decisions would provide clues regarding the processes involved. We first dividing the ERP responses according to whether they had been elicited by the first or the second time an item had been presented. A late positivity of greater amplitude was observed in conjunction with the second relative to the first presentation of the stimuli for both older and younger adults. These results replicate a number of studies (e.g., Rugg et al., 1994) that have demonstrated age-invariance in what is commonly referred to as the ‘‘repetition effect.’’ It was when we compared ERP responsivity during source discrimination that dissociable age-related response patterns emerged. In the case of young adults, the amplitude of the late positivity was reliably greater for study words, the designated targets, relative to lag words which, although familiar, were to be ignored. In fact, the younger adults seemed able to inhibit the expected ERP repetition response to the lag words. Older adults, on the other hand, produced ERP late positivities to lag words that were as great or greater in amplitude than those they produced for target material. Such dissociations between ERP responsivity and target discriminability are not typically noted in standard old/new discrimination tasks. However, placing the familiarity and the ‘‘targetness’’ of the stimuli in opposition has allowed us to observe dissociations between automatic and controlled processing as they relate to the accomplishment of task goals. For older adults in this study, the ERP data indicate a poorly controlled reactivity to nontarget lag words. While it is true that the older adults made more source error we cannot assume that the heightened reactivity was due to a failure in recognition. ERPs were averaged only for trials on which subjects had, in fact, made a correct behavioral response. So, even when correctly rejecting the lag item as a target, the heightened reactivity was observed. On the basis of this dissociation, we propose that older adults may be less able than the young to inhibit their physiological reactivity to the recently presented information even when they are able to recover sufficiently to avoid actually making a source error. What needs to be explained, however, is why the younger adults ERPS AND SOURCE MONITORING 405 did not show the same degree of reactivity to the recently repeated lag words. It would be hard to argue that words repeated after a lag of six intervening items would engender no sense of familiarity in the young adults. One would have to assume, therefore, that the younger adults are better able to inhibit their physiological reaction to the nontarget lag words despite the fact that these words would be perceived as familiar. In an earlier study (Dywan and Murphy, 1996) we demonstrated that young adults appeared to inhibit the initial processing of distracting phrases which had been interspersed in paragraph length text. While the younger adults appeared more able to ignore the distracting material when reading the text and again when their comprehension was tested, they were nonetheless better able to recognize that material later during a surprise test of recognition. We concluded that flexibility in the control of attention is central to the ability to control response tendencies. The centrality of attentional capacity in the control of inhibitory function has also been demonstrated by Engle, Conway, Tuholski, and Shisler (1995) with respect to negative and positive priming. On the basis of such data, we hypothesize that it is attentional control, rather than a decline in inhibitory processes per se that might account for the ERP effects during source monitoring. The centrality of attentional resources has, in fact, been demonstrated with respect to source memory. Jacoby, Woloshyn, and Kelley (1989) asked young adults to make fame judgments under full and divided attention conditions. As in the present study, young adults were quite good at distinguishing between sources of familiarity. That is, they were able to recognize when a nonfamous name had been repeated in the experimental setting and did not falsely attribute that familiarity to fame. However, under divided attention conditions, their ability to make this source discrimination was reduced and they made significantly more source error, i.e., they misattributed the familiarity of the repeated nonfamous names to their being famous. Thus, asking young adults to do the lag task under divided attention conditions should also increase source error since the tasks are structurally similar. Our question, however, was whether this increased source error rate would be accompanied by ERP patterns similar to those observed in Study 1. That is, would distracting young adults impair their ability to inhibit a physiological response to the salience of the recently encountered lag words. Such data would highlight the importance of attentional capacity in enabling inhibitory control to occur. It would also highlight the role of attentional capacity, as opposed to mnemonic strength, per se, in source memory performance. STUDY 2 Study 2 was designed to replicate the results of the first experiment and to more directly examine the role of attentional capacity in making source monitoring decisions. Thus, in this second experiment, we compared the performance of new groups of older and younger adults on the same para- 406 DYWAN, SEGALOWITZ, AND WEBSTER digm as was used in Study 1 but also included a second group of young adults who were asked to make source monitoring decisions under divided attention conditions. We assumed that distracting young adults would increase their source error to more closely match that of the older adults. We were hoping that ERPs recorded during source monitoring would reveal whether reducing attentional capacity would also affect inhibitory control in the younger adults. Method Participants were 19 active older adults from the local community (M age ⫽ 69.6 years) and a control group of 15 younger adults (M age ⫽ 20.3 years). The older adults had a marginally greater level of education (M ⫽ 15.3 years) than the younger adults (M ⫽ 14.3 years), t(32) ⫽ 1.94, p ⫽ .06. A second group of younger adults (M age ⫽ 20.1 years; M years of education ⫽ 14.0) was recruited to serve in the dual-task condition. In this study, scores were available from the Vocabulary subtest of the Wechsler Adult Intelligence Scale—Revised (WAIS-R; Wechsler, 1987). The young groups did not differ in vocabulary (M ⫽ 44.7 vs M ⫽ 47.9 for the young dual-task group and the young control group, respectively). However, the older adults (M ⫽ 57.53) produced higher vocabulary scores than either the young dual-task group, t(32) ⫽ 6.26, p ⬍ .001, or the young controls, t(32) ⫽ 4.04, p ⬍ .001. Thus, these older adults could not be considered disadvantaged relative to the younger adults with respect either to education or general verbal ability. As in the previous study, initial screening eliminated participants with a history of substance abuse, cerebrovascular or cardiovascular accident, psychiatric disorder, traumatic brain injury, nonnormative levels of cognitive decline, or health problems that would interfere with normal performance. Community volunteers were paid a nominal fee to cover incidental expenses, if any, and those young adults who were undergraduates received course credit for their participation. Stimuli Stimuli consisted of the same word lists as were used in Study 1. The only addition was an auditory tape consisting of a random series of digits from 1 to 9 that occurred approximately every 2 seconds. This was used as a distractor task for the young adults in the dual-task condition. Procedure All participants were presented with the stimuli in exactly the same way as in Study 1, except during the recognition phase of the study. Young adults in the dual-task group were asked to listen to a string of random numbers between 1 and 9 and to press a mouse key with the index finger of their 407 ERPS AND SOURCE MONITORING TABLE 2 Proportion of Words Judged to Be from the Study List (pStudy) and Latency of Behavioral Response (RT) by Type of Word for Young Adults, Young Adults in the Dual-Task Condition, and Older Adults (Study 2) Young adult Type of word Study M SD Lag M SD Foil M SD Dual-task Older adult pStudy RT (ms) pStudy RT (ms) pStudy RT (ms) .56 .17 897 90 .51 .13 1070 214 .46 .22 1108 239 .17 .11 889 129 .30 .20 1134 197 .43 .28 1201 274 .08 .07 1015 355 .17 .12 958 531 .11 .12 1107 479 Note. Response times are for correct decisions only. nondominant hand whenever they heard 3 odd numbers in a row. To ensure uniformity of response, all participants used the index and middle fingers of their dominant hand to press adjacent computer keys to designate words in the lag task as either study ‘‘yes’’ or nonstudy ‘‘no.’’ The equipment was arranged to easily accommodate the handedness of the participant. It was emphasized that both the digit-tracking task and the source monitoring task were important and that participants should divide their attention equally between them. ERP recording procedures and criteria for acceptance were the same as in Study 1 except that a 200-ms baseline was used.5 Rejection of subjects on the basis of too few artifact-free trials reduced the size of the older adult group to 14, the young control group to 15, and the young dual-task group to 10 for the ERP analyses. Results and Discussion The behavioral choices and response times of the three groups are presented in Table 2. For each type of response (behavioral, RT, and ERP) data from all three groups were initially included in a single analysis. The full analysis of behavioral source monitoring decisions indicated no significant effect for group, F(2, 46) ⫽ .92, ns, but the effect of stimulus type, F(2, 92) ⫽ 118.96, p ⬍ .001, ⫽ 72, and the group by stimulus type interaction, F(4, 92) ⫽ 9.57, p ⬍ .001, η 2 ⫽ .30, were reliable. Comparing groups with respect 5 Lab procedures regarding prestimulus baseline were changed in the year between the two studies. The longer prestimulus baseline does not change any of the waveshapes, only the Xaxis location. 408 DYWAN, SEGALOWITZ, AND WEBSTER to response time indicated only a main effect for group, F(2, 46) ⫽ 3.25, p ⫽ .048, η 2 ⫽ .12, but there was no effect for stimulus type nor was there a group by stimulus type interaction.6 In the following sections we describe the secondary planned analyses in which we specifically compare the performance of the young controls with the older adults to determine the degree to which the results of the first study were replicated. We then report the relationship between the young controls and the young adults in the dual task condition. Finally, we report the relationship between the dual task group and the older adults. Behavioral and RT Data Older adults vs young controls. We first compared the performance of the older adults and young controls who had performed under the same conditions as in Study 1. These data were subjected to a 2 ⫻ 3 repeated measures analysis of variance (ANOVA) with group (older adults vs young controls) as the between-group factor and stimulus type (study word vs lag word vs foil) as the within-group factor. As in Study 1, the older adults were slightly more likely to designate items as targets (M ⫽ .33) than the younger adults (M ⫽ .27), but again, this difference was not reliable, F(1, 32) ⫽ 1.48, ns. There was the expected main effect for stimulus type, F(2, 64) ⫽ 90.03, p ⬍ .001, η 2 ⫽ .74, such that the probability of correctly responding ‘‘yes’’ to a study word (M ⫽ .51) was greater than the probability of responding ‘‘yes’’ to either the lag words (M ⫽ .30) or the foils (M ⫽ .10). There was also a group by stimulus type interaction, F(2, 64) ⫽ 17.32, p ⬍ .001, η 2 ⫽ .35, such that the tendency to say ‘‘yes’’ to study items was similar between older (M ⫽ .46) and younger (M ⫽ .56) adults, as was the tendency to say ‘‘yes’’ to foils (M ⫽ .11 vs M ⫽ .08 for older and younger adults, respectively). Of most importance, the older adults were again more than twice as likely to designate lag words as study words (M ⫽ .43) compared to the younger adults (M ⫽ .17), thus replicating, almost exactly, the behavioral results reported in Study 1. RTs to correct acceptance of study items and correct rejection of lag and foil items (Table 2) were submitted to a 2 ⫻ 3 group (older vs younger adults) by stimulus type (study correct vs lag correct vs foil correct) ANOVA. In 6 RTs in Study 2 were consistently longer for all groups than they had been in Study 1. We believe this occurred because of a change in response parameters. In Study 1 participants used the index finger from one hand for targets and the index finger from the other hand for nontargets. In Study 2, the dual-task group needed their nondominant hand for pressing the mouse button when the auditory target sequence appeared, so all participant groups used the index and middle fingers of the dominant hand for their memory task responses. Presumably, a less differentiated spatial response would be more difficult and would lead to the slightly longer RTs. However, since our goal is to compare within-subject responses to different stimulus conditions, a general slowing of RTs does not present an interpretive dilemma. ERPS AND SOURCE MONITORING 409 general, behavioral responses were slower for the older adults (M ⫽ 1139 ms) than for the younger adults (M ⫽ 934 ms), F(1, 32) ⫽ 7.39, p ⫽ .01, η 2 ⫽ .19. However, unlike the previous study, RTs to various classes of item were not reliably different, F(2, 64) ⫽ .49, ns. There was also no group X stimulus type interaction with respect to RT, F(2, 64) ⫽ .21, ns, indicating that speed of response differences between the groups did not reflect the particular difficulties the older adults were having in making correct source judgements as might have been inferred from the RT data of Study 1. Young adults: Control vs dual-task conditions. The young adults in the dual-task condition performed more like the older adults than like their agematched peers. They were slightly more likely to designate items as study words in general (M ⫽ .32) than the young controls (M ⫽ .27) but, as was the case for the age-based comparisons, this difference was not reliable, F(1, 28) ⫽ 1.70, ns. There was, of course, an effect of stimulus type, F(2, 56) ⫽ 123.07, p ⬍ .001, η 2 ⫽ .81, with both groups more likely to say ‘‘yes’’ to study words (M ⫽ .54) relative to lag words (M ⫽ .23) and foils (M ⫽ .13). There was also a group by stimulus-type interaction, F(2, 56) ⫽ 6.22, p ⬍ .025, η 2 ⫽ .18, such that the tendency to say ‘‘yes’’ to study items was very similar between the young controls (M ⫽ .56) and the dual-task group (M ⫽ .51). However, like the older adults, the dual-task group was more likely to say ‘‘yes’’ to the lag words (M ⫽ .30) than were the young controls (M ⫽ .17), thus indicating that distraction at test does result in more source error. The young dual-task subjects in this study were also more likely to produce false positive responses to the foils (M ⫽ .17) relative to young controls (M ⫽ .08), which was not true of the older adults in the first or second study. Comparing the reaction times for the younger adults in the dual task vs the control condition indicated that the dual task group (M ⫽ 1054 ms) was a little slower than the control group (M ⫽ 934 ms) but this difference was not reliable, F(1, 28) ⫽ 2.23, ns. There was no main effect for stimulus type nor was there a reliable group by stimulus type interaction. Thus, the young adults in the dual task condition differed from the young controls in their tendency to make source errors but not in response times. Young dual-task subjects vs older adults. Comparing the behavioral responses of young adults in the dual-task condition to those of the older adults indicated no main effect for group, F(1, 22) ⫽ .05, ns. There was an effect of stimulus type, F(2, 64) ⫽ 54.35, p ⬍ .001, η 2 ⫽ .63, with both groups more likely to say ‘‘yes’’ to study words (M ⫽ .48) relative to lag words (M ⫽ .37) and foils (M ⫽ .14). There was also a group by stimulus-type interaction, F(2, 64) ⫽ 4.77, p ⬍ .05, η 2 ⫽ .13, such that the tendency to say ‘‘yes’’ to study items was very similar between the young dual task group (M ⫽ .51) and the older adults (M ⫽ .46) as was their tendency to say ‘‘yes’’ to foils (M ⫽ .17 vs M ⫽ .11 for young dual-task and older groups, respectively). However, despite being distracted, the young dual- 410 DYWAN, SEGALOWITZ, AND WEBSTER task group did not make quite as many source errors (M ⫽ .30) as did the older adults (M ⫽ .43). Thus, distraction rendered the young dual-task group significantly more vulnerable to source error than the young controls but not quite as vulnerable to source error as the older adults in the standard experimental condition. Comparing the reaction times for the younger adults in the dual task group with those of the older adults indicated that the dual task group (M ⫽ 1054 ms) was a little faster than the older adult group (M ⫽ 1139 ms) but this difference was not reliable, F(1, 32) ⫽ .91, ns. There was no main effect for stimulus type nor was there a reliable group by stimulus type interaction. Summary of behavioral and RT data. As in Study 1, the older adults were far more likely to make source errors than the young adults or even the young adults in the dual-task group. However, the young adults in the dual-task group were significantly more likely to make source errors than the young controls. Thus, the results of Study 1 have been replicated with respect to the behavioral data and we have established that reducing attentional capacity at test is sufficient to disrupt source monitoring performance in young adults even when initial encoding conditions are not disrupted. Moreover, deficits in attentional capacity, whether they were associated with age or dual-task conditions in young adults, had the effect of slowing performance overall; i.e., both the young dual-task group and the older adults were slower than the young controls. However, speed of response did not interact with type of stimulus or with group by stimulus-type interactions so that speed of processing is unlikely to be central to the source monitoring deficits observed here. ERP Data: The Repetition Effect ERPs averaged over repeated versus nonrepeated words are presented in Fig. 5 for the three groups.7 Visual inspection indicates that all groups produced a heightened positivity for the old words relative to the new words. Graphs depicting area under the curve at 50-ms intervals are represented in Fig. 6. The shaded areas represent those intervals in which the difference in ERP amplitude between old and new items had p values less than .1 (range p ⫽ .09 to .0001). The area from 350 to 750 ms encompassed the region of maximal differentiation with respect to the simple repetition effect at each site for all groups.8 As with the behavioral and RT data, the ERP data were initially analyzed in a full model ANOVA followed by the results from the planned comparisons. All data was subjected to Huynh–Feldt corrections for multiple comparisons when appropriate. The 200-ms baselines used in Study 2 are represented only to ⫺100 ms in the figures. The delayed onset of the late positivities in Study 2 relative to Study 1 are consistent with the delayed RTs, both due, we assume to the more difficult unimanual response mode used in Study 2. 7 8 ERPS AND SOURCE MONITORING 411 FIG. 5. Grand average ERPs generated by young controls, young adults in the dual-task condition, and older adults to words previously seen (old) and words not previously seen (new) irrespective of context (Study 2). FIG. 6. ERP amplitude at 50-ms intervals relative to 200-ms prestimulus baseline as elicited by previously seen words (old) and words not previously seen (new) irrespective of context for young controls, young adults in the dual-task condition, and older adults (Study 2). 412 DYWAN, SEGALOWITZ, AND WEBSTER With respect to ERP analyses of simple repetition effects, there was a significant difference involving stimulus type, F(1, 36) ⫽ 27.06, p ⬍ .001, η2 ⫽ .43. The amplitude of the late positivity was greater for previously seen words but there was no main effect for group nor any group by stimulus type interactions. With respect to the amplitude of ERPs during source monitoring, there was again no main effect for group, F(2, 36) ⫽ .34, ns, but there was an effect for stimulus type, F(2, 72) ⫽ 7.02, p ⬍ .01, η 2 ⫽ .16, and a significant group by stimulus type interaction, F(4, 72) ⫽ 2.68, p ⬍ .05, η 2 ⫽ .13, the details of which will be examined below with respect to the questions this study was designed to address. Whether examining ERP repetition effects or source monitoring effects, amplitude differences were observed as a function of site, F(2, 72) ⫽ 23.59, p ⬍ .001, η 2 ⫽ .40 and F(2, 72) ⫽ 21.11, p ⬍ .001, η 2 ⫽ .37, for repetition and source-monitoring effects, respectively. There was also a significant site by group interaction for repetition effects, F(4, 72) ⫽ 27.37, p ⬍ .001, η 2 ⫽ .36, and for source monitoring, F(4, 72) ⫽ 7.47, p ⬍ .001, η 2 ⫽ .29. Amplitude differences across site and group will also be described in the context of the various planned comparisons to follow. However, these site by group interactions were unrelated to stimulus type so are not central to the main hypotheses being addressed. Repetition Effect Young adults vs older adults. The late positivities calculated from 350 to 750 ms were entered into a 2 ⫻ 2 ⫻ 3 ANOVA comparing groups (younger adults vs older adults), stimulus types (‘‘previously seen’’ vs ‘‘not previously seen’’), and sites (Fz, Cz, and Pz). Results indicate that there was no overall effect for group, F(1, 27) ⫽ .92, ns. There was a significant effect for stimulus type, F(1, 27) ⫽ 20.28, p ⬍ .001, η 2 ⫽ .43. Previously seen words (M ⫽ 1.98 µV) elicited late slow potentials that were generally more positive than those elicited for new words (M ⫽ .41 µV). There was also a main effect for site, F(2, 54) ⫽ 19.65, p ⬍ .001, η 2 ⫽ .42, indicating that the positivity at Fz (M ⫽ 2.10 µV) tended to be greater relative to that at Pz (M ⫽ 1.20 µV) or Cz (M ⫽ .29 µV). Group interacted with site, F(2, 54) ⫽ 21.43, p ⬍ .001, η 2 ⫽ .44, reflecting older adults’ tendency to produce the greatest positivities at Fz (M ⫽ 3.58 µV) relative to Cz (M ⫽ .55 µV), or Pz (.69 µV). Young adults did not show the hyperfrontality noticed in older groups (M at Fz ⫽ .72 µV; M at Cz ⫽ .05 µV; M at Pz ⫽ 1.67 µV) and, in fact, produced the greatest amplitude at the posterior site. There was no group by stimulus type interaction, F(1, 27) ⫽ .56, ns, indicating that the difference in late positivity to old versus new words was not significantly greater for the younger relative to the older adults. There was also no relationship between type of stimulus and site or between type of stimulus and the group by site interaction. ERPS AND SOURCE MONITORING 413 These analyses indicate that the old/new effect was evident in both groups, that frontal sites produced a marginally greater positivity than more posterior sites, and that this occurred more for the older relative to the younger participants. However, site effects were not related to stimulus type (previously seen vs not previously seen). The site effects likely represent general age changes in ERP reactivity and do not constitute a specific response to item familiarity. Young adults vs the young dual-task group. Using the same format for analyses as described in the previous section, we found no overall effect for group, F(1, 23) ⫽ .57, ns. There was a significant effect for stimulus type, F(1, 23) ⫽ 39.25, p ⬍ .001, η 2 ⫽ .63. Previously seen words (M ⫽ 1.92 µV) elicited late slow potentials that were generally more positive than those elicited by new words (M ⫽ .33 µV). There was also a main effect for site, F(2, 46) ⫽ 9.19, p ⬍ .001, η 2 ⫽ .29, indicating that the positivity at Pz tended to be greater (M ⫽ 1.71 µV) relative to Fz (M ⫽ 1.41 µV) or Cz (M ⫽ .26 µV) as seems to be the case for younger adults in general and there was no interaction between group and site, F(2, 46) ⫽ 2.71, ns. Of most interest, there was no group by stimulus type interaction, F(2, 46) ⫽ 1.90, ns., indicating that distraction did not produce a difference in the size of the repetition effect. None of the other interactions (condition by site or group by condition by site) approached significance. These data suggest that the simple repetition effect, at least as measured in this paradigm, is based on fairly automatic information processing routines. Young dual-task group vs older adults. Using the same format for analysis, we found no overall effect for group, F(1, 22) ⫽ .01, ns. There was a significant effect for stimulus type, F(1, 22) ⫽ 9.64, p ⫽ .005, η 2 ⫽ .30. Previously seen words (M ⫽ 2.24 µV) elicited late slow potentials that were generally more positive than those elicited for new words (M ⫽ .96 µV). There was also a main effect for site, F(2, 44) ⫽ 28.15, p ⬍ .001, η 2 ⫽ .56, indicating that the positivity at Fz (M ⫽ 3.12 µV) tended to be greater relative to Cz (M ⫽ .56 µV) or Pz (M ⫽ 1.14 µV). There was also a group by site interaction, F(2, 44) ⫽ 5.46, p ⫽ .008, η 2 ⫽ .20, as seems typically to be the case when comparisons are made across rather than within age groups. There was, however, no group by stimulus-type interaction, F(1, 22) ⫽ .01, ns., indicating that the repetition effect was similar in both the dual-task and older adult groups. No other interactions approached significance. The ERP Source-Monitoring Effect Averaged ERPs for study words, lag words, and foils elicited during the test phase of the experiment are presented in Fig. 7. For the older adults, ERPs to study and lag words were almost overlapping in the initial portions of the wave with a slightly greater positivity for study words at the frontal site. The older adults produced their most distinct response to the two types 414 DYWAN, SEGALOWITZ, AND WEBSTER FIG. 7. Grand average ERPs generated by young controls, young adults in the dual-task condition, and older adults to study words, lag words, and foils (Study 2). of stimuli between 700 and 800 ms, but the direction of differentiation was opposite to that of the younger adults, i.e., for older adults the lag words elicited the highest amplitude positivities. The young adults from the dualtask group produced the greatest differentiation between study and lag words from about 350 to 400 ms. These distracted young adults showed the same tendency as observed in the older adults to produce higher amplitude late positivities to the nontarget but more recently presented lag words. Thus, on the basis of visual inspection, it would appear that reducing the attentional capacity of young adults leads to the same problems with inhibitory control that we have seen in the older adults in both Study 1 and Study 2. Again, the shaded areas in Fig. 8 represent those intervals in which the difference in ERP amplitude between study items and lag items had p values less than .1 (the range is p ⫽ .08 to .001). To compare groups, the areas from 350 to 800 ms were combined in order to encompass the areas of maximal differentiation for all three groups. As described above, these late positivities were entered into a 3 ⫻ 3 ⫻ 3 ANOVA comparing groups (older adults vs young controls vs young dual task), stimulus type (study words vs lag words vs foils), and sites (Fz, Cz, and Pz). For ease of presentation, the secondary ANOVAs are presented in which we examine specific hypotheses. ERPS AND SOURCE MONITORING 415 FIG. 8. ERP amplitude at 50-ms intervals relative to 200-ms prestimulus baseline as elicited by lag words and study words for young controls, young adults in the dual-task condition, and older adults (Study 2). Shaded areas represent intervals in which the difference in amplitude between study and lag items had p values of less than .1 (range: p ⫽ .080 to .001). Young adults vs older adults. Comparing the late positivities calculated on the region from 350 to 800 ms, we found no main effect of group, F(1, 27) ⫽ .41, ns. There was, however, a main effect for stimulus type, F(2, 54) ⫽ 7.04, p ⫽ .002, η 2 ⫽ .21. ERP amplitude to foils was generally less positive (M ⫽ .54) than for either lag words (M ⫽ 1.64) or study words (M ⫽ 2.30). There was a main effect for site, F(2, 54) ⫽ 20.07, p ⬍ .001, η 2 ⫽ .43, with higher amplitude at Fz (M ⫽ 2.47 µV) than at Cz (M ⫽ .65 µV) or Pz (M ⫽ 1.35 µV). There was also a group by site interaction, F(2, 54) ⫽ 16.99, p ⬍ .001, η 2 ⫽ .39, indicating a tendency for increased frontality in older adults. The late positivity for older adults was greatest at Fz (M ⫽ 3.73 µV) relative to central, Cz (M ⫽ .78 µV), and posterior sites, Pz (M ⫽ .83 µV). The amplitude of the ERPs in this same interval for the younger adults was highest at the most posterior site, Pz (M ⫽ 1.84 µV), relative to the frontal site, Fz(M ⫽ 1.30 µV), or the central site, Cz (M ⫽ 0.53 µV). What is of most interest, however, was the group by stimulus type interaction, F(2, 54) ⫽ 3.40, p ⫽ .04, η 2 ⫽ .11, confirming that the older adults did tend to produce a greater positivity across sites to the lag words (M ⫽ 2.46 µV) relative to study words (M ⫽ 1.89 µV) or foils (M ⫽ .98 416 DYWAN, SEGALOWITZ, AND WEBSTER µV), while the younger adults produced the greatest positivity to study words (M ⫽ 2.68 µV) relative to lag words (M ⫽ .86 µV) or foils (M ⫽ .12 µV). Group effects for stimulus type did not interact with site (see footnote 3). These results provide a full replication of the ERP source monitoring effects of Study 1. Young adults vs young dual-task group. Again we found no main effect of group, F(1, 23) ⫽ .46, ns. There was, however, a main effect for stimulus type, F(2, 46) ⫽ 6.10, p ⫽ .004, η 2 ⫽ .21. ERP amplitude to foils was generally less positive (M ⫽ .52) than for either lag words (M ⫽ 1.73) or study words (M ⫽ 2.30). There was a main effect for site, F(2, 46) ⫽ 6.83, p ⫽ .003, η 2 ⫽ .23, such that the amplitude at Cz is smaller (M ⫽ .74 µV) than at either Fz (M ⫽ 1.89 µV) or Pz (M ⫽ 1.91 µV) but no group by site interaction, F(2, 46) ⫽ 1.56, ns. Of most interest was a significant group by stimulus type interaction, F(2, 46) ⫽ 5.22, p ⫽ .009, η 2 ⫽ .19, confirming that, like the older adults, the young adults in the dual-task group produced a greater positivity across sites to the lag words (M ⫽ 3.02 µV) relative to study words (M ⫽ 1.73 µV) or foils (M ⫽ 1.12 µV), while the young controls produced the greatest positivity to study words (M ⫽ 2.68 µV) relative to lag words (M ⫽ .86 µV) or foils (M ⫽ .12 µV). Group effects for stimulus type did not interact with site (see footnote 3). These results indicate that reducing attentional capacity in younger adults results in an ERP response during source monitoring that is remarkably similar to that seen in older adults under full attention conditions. Young dual-task adults vs older adults. Our next question was whether the young adults in the dual-task condition produced the same degree of reversal in their ERP response as that seen in the older adults. Behaviorally, the young dual-task group made significantly more source monitoring errors than the young controls but significantly fewer than the older adults and it would be interesting to see whether these differential accuracy levels might be reflected in group differences in the ERP data as well. The overall ERP late positivity did not differ as a function of group, F(1, 22) ⫽ .04, ns. There was a main effect for stimulus type, F(2, 44) ⫽ 4.06, p ⬍ .05, η 2 ⫽ .16. ERP amplitudes to foils were generally less positive (M ⫽ 1.04) than for study words (M ⫽ 1.82), which in turn were less positive than for the lag words (M ⫽ 2.69). There was a main effect for site, F(2, 44) ⫽ 23.01, p ⬍ .001, η 2 ⫽ .51, such that the amplitude at Fz (M ⫽ 3.33 µV) was larger than at either Cz (M ⫽ 0.90 µV) or Pz (M ⫽ 1.33 µV). There was also a group by site interaction, F(2, 44) ⫽ 4.49, p ⬍ .05, η 2 ⫽ .17. The older adults showed a markedly greater amplitude at the frontal site, Fz (M ⫽ 3.73 µV) relative to central, Cz(M ⫽ .78 µV), or parietal, Pz (M ⫽ .834 µV) sites while the young dual-task group showed a more even degree of positivity across sites: Fz (M ⫽ 2.77 µV); Cz (M ⫽ 1.06 µV); Pz (M ⫽ 2.03 µV). What is of most interest with respect to this comparison is that even though we have groups of markedly different ages, there is no group ERPS AND SOURCE MONITORING 417 by stimulus type interaction, F(2, 44) ⫽ .19, ns. Also, site did not interact with other experimental variables (see footnote 3). Thus, it would seem that both the older adults and the young dual-task group produced greater amplitude late positivities to the repeated lag words than to the target study words. This suggests that a reduction in attentional capacity is linked in some way to the ability to inhibit neurophysiological reactivity to nontarget information. Interpreting Differential Amplitudes in the Late Positivity ERP Young adults in the standard lag paradigm consistently produced higher amplitude late positivities to target words but not to lag words or foils. From such data, one could conclude that for young adults, the amplitude of the late positivity corresponds with the salience of goal relevant information. That is, the late positivity would reflect the targetness of the stimulus rather than its familiarity per se. Older adults and young adults in the dual-task conditions produced high-amplitude late positivities to the more immediately repeated lag words than they did to the target words from the study list. It would seem, therefore, that reduced attentional capacity, whatever its source (i.e., aging or divided attention), allows the nervous system to be more easily overwhelmed by stimulus properties that could affect response tendencies. As we have seen, this reactivity does not automatically lead to incorrect behavioral decisions; i.e., all ERP responses were averaged across trials on which participants had made a correct choice. It would appear, therefore, that the ERP late positivity represents the degree to which the information appears salient to the individual. We suggest that even though older adults are able to overcome this automatic reactivity when making a behavioral response, the physiological experience would, nonetheless, predispose them to a higher rate of source error. There is, however, an alternative hypothesis. It may be that a reduced ability to recollect information leaves subjects confused about the source of item familiarity and that this confusion or ambiguity makes the lag words salient for older adults and for young adults in the dual-task condition. To compare these models, we examined the ERP late positivities to lag items that occurred when participants did, in fact, make a lag error. If lag errors occur because of overreactivity to the most recently presented information (the inhibitory control hypothesis) then the amplitude on error trials (when lags are responded to as study words) should be greater than the amplitude on trials on which the participant is able to overcome this reactivity and respond appropriately. However, if the late positivity to lag items in the older adult and young dual task groups is due to impaired recollection (mnemonic ambiguity hypothesis), then the amplitude of these late positivities should be lower when lag errors (i.e., false positives) occur.9 In such cases, partici9 We thank Andrew Young and Karalyn Patterson for drawing our attention to this point. 418 DYWAN, SEGALOWITZ, AND WEBSTER FIG. 9. Grand average ERPs as elicited on false positive trials (lag error) and on trials on which the lag words were correctly rejected (lag correct). These ERPs were generated by eight older adults who made enough artifact-free lag errors to form an acceptable average. pants would not try to counter the experience of familiarity by trying to recollect source but would instead ‘‘go with the flow’’ and assimilate familiarity with targetness. There should be little ambiguity in such responses and so we should not see the exaggerated late positivity that we see when lagbased familiarity is countered as it is on correct trials. It is difficult to compute ERP averages across error trials as there are usually fewer of them than correct trials and once trials with movement artifact are eliminated, there are too few trials on which to calculate a stable ERP. There were, however, eight older adults in Study 2 who made a sufficient number of artifact-free lag errors to allow for the calculation of an ERP late positivity. In Fig. 9 ERPs collected from false positive trials (lag error) have been plotted along with those from trials on which the lag word was correctly rejected (lag correct). Visual inspection indicates that when subjects make a lag error (i.e., when they mistakenly categorize the lag word as a study word) they do produce a heightened positivity in the early portion of the waveform that is greater than the positivity that occurs when the lag word is correctly rejected. However, a correctly rejected lag word continues to elicit a heightened late positivity in the later portion of the waveform that is greater than that which occurred to the lag errors. To test the statistical ERPS AND SOURCE MONITORING 419 FIG. 10. ERP amplitude at 50-ms intervals relative to 200-ms prestimulus baseline as elicited on false positive trials (lag error) and on trials on which the lag words were correctly rejected (lag correct). Shaded areas represent intervals in which the difference in amplitude between study and lag items had p values of less than .1 (range: p ⫽ .090 to .002). reliability of this effect, we formed two components: an early positivity (200 to 650 ms) and a later positivity (650 to 900 ms) (see Fig. 10). These were submitted to a 2 ⫻ 2 ⫻ 3 ANOVA comparing stimulus type (lag correct vs lag error), component (early vs late), and site (Fz, Cz, Pz). Results indicated that there were no main effects for stimulus type or component. There was, however, a main effect for site, F(2, 14) ⫽ 11.17, p ⬍ .001, η2 ⫽ .62, such that the overall amplitude at Fz (M ⫽ 4.24 µV) was greater than at Cz (M ⫽ 2.09 µV) or Pz (M ⫽ 1.99 µV) showing the same pattern of hyperfrontality that was observed in the full group of older adults. The interaction between stimulus type and component was also significant, F(1, 7) ⫽ 31.52, p ⬍ .001, η 2 ⫽ .82 confirming what seems obvious from visual inspection. When participants responded to lag words as though they were the targeted study words, the early component of the waveform (M ⫽ 3.94 µV) was considerably more positive than when the lag word was correctly rejected (M ⫽ 2.20 µV). The late components of the waveforms are 420 DYWAN, SEGALOWITZ, AND WEBSTER significantly different in the opposite direction. The positivity that occurs for a lag error is not sustained (M ⫽ .98 µV) but increases dramatically when the lag word is correctly rejected (M ⫽ 3.97 µV). Unfortunately, these data do not unambiguously distinguish between the two hypotheses (inhibitory control vs mnemonic ambiguity). What is clear, however, is that considerable reactivity accompanies the initial processing of lag words that are incorrectly categorized as study words. It may be that the early reactivity to these words, brought about perhaps by their recency, leads participants to respond as though to a target. The reactivity represented in the second component could reflect a later occurring conscious decision process (cf. Osterhout, McKinnon, Bersick, and Corey, 1996; Wilding and Rugg, 1996). Summary Behaviorally, the performance of the older and younger adults in the standard lag paradigm provided a full replication of the results from Study 1. The older adults made more source errors than the younger adults when presented with lag words despite relatively similar accuracy levels for study words and for foils. Also, the results of divided attention at test were as predicted. Young adults in the dual-task condition made significantly more source errors than did young adults in the full attention condition. As was the case in Study 1, older adults had prolonged behavioral reaction times relative to the younger adults. Young adults in the dual task condition also showed the same slowing of behavioral reaction indicating that reduced information processing speed can be the result of as well as the cause of reduced attentional resources. So, it may be that older people are slower because they have reduced attention resources as opposed to having reduced attentional resources because they are slower (cf. Salthouse, 1991). The relationship between RT and source error was not stable. In Study 1 there was an interaction between response latency, type of item, and group such that older adults were slowed more than younger adults when confronted with the lag items at test compared to study words or foils. This was not found in Study 2 for either the older or younger adults in the full attention condition or for the dual-task group. Thus, speed of processing is unlikely to be central to the source monitoring deficits observed here. As for the ERP data, stimuli previously seen in the experimental context elicited greater amplitude late positivities than did those elicited by new stimuli. This ERP repetition effect occurred to the same extent for older and younger adults in the full-attention condition and for the young adults in the dual-task condition. We found, however, that when the task required participants to discriminate between sources of familiarity, the same agerelated dissociations in ERP response occurred as was seen in Study 1. Young adults in the full-attention condition produced a high-amplitude late ERPS AND SOURCE MONITORING 421 positivity to the study words, which served as the target stimuli, but not to the lag words or the foils. This was not the case for the older adults who not only produced a late positivity to the lag words but produced one that was larger than for the study words. To our knowledge, this age-related dissociation in ERP response to different sources of familiarity has not been reported before. We assumed that the ability to distinguish between sources of familiarity was more dependent on higher order, conceptually driven processing than was the ability to make a simple old/new discrimination and as such should be more disrupted under dual-task conditions. Both the behavioral and ERP response of the young adults in the dual-task condition supported this view. Disrupting attention at test had no effect on ERP indices of old/new discrimination. However, young adults in the dual-task condition were more likely than controls to make source error and they produced a late positivity to the nontarget lag words that was of higher amplitude than that produced to the targeted study words, a response remarkably similar to that of older adults. Even though the ERP reactivity to nontarget information does not cause source error (i.e., it occurred on correct as well as incorrect trials), it would be reasonable to assume that the physiological reactivity that occurs to the nontarget information could influence a behavioral decision. In fact, we proposed that the greater the physiological reactivity, the harder it would be to oppose. Examining the ERP response that occurred to incorrect lag decisions demonstrated that these were significantly more positive in the initial portion of the waveform than was the ERP response for those trials on which the lag word was correctly rejected. Thus, that initial burst of response to the familiarity of the recently repeated lag word seemed to have the potential to overwhelm higher order decision processes and lead to increased levels of source error. General Discussion The observation that older adults are more prone to source error than younger adults was consistent with observations made across a broad range of experimental procedures (e.g., Dywan & Jacoby, 1990; Dywan et al., 1994; Jennings & Jacoby, 1993, 1997; Schacter, Osowiecki, Kaszniak, Kihlstrom, & Valdiserri, 1994; Spencer & Raz, 1995; see also, Spencer & Raz, 1995, for a review). Similarly, the high source error rate for young adults in the dual-task condition has been demonstrated in previous work (e.g., Jacoby et al., 1989). Dual-task instructions are typically found to interfere with intentional, controlled aspects of remembering while leaving more automatic influences intact (e.g., Gruppuso, Lindsay, & Kelley, 1997; Jacoby, Toth, & Yonelinas, 1993). We believe that it is this imbalance between automatic and controlled processes that predisposes an individual to make source error. Comparing ERP amplitudes elicited during the presentation of new items 422 DYWAN, SEGALOWITZ, AND WEBSTER (foils and first-presentation lag words) with those elicited to items seen before irrespective of context (e.g., study words and second-presentation lag words) we found patterns similar to those reported by others (Hamberger & Friedman, 1992; Friedman et al., 1992, 1993a; Rugg et al., 1994) in that repetition of the stimuli elicited a higher late positivity to previously seen words and this occurred for both older and younger participants. Not only were older participants as likely to demonstrate the repetition effect but they produced a late positivity of similar amplitude and somewhat greater duration than the younger adults, thus replicating Friedman et al. (1993b) and Karayanidis et al. (1993). The ERP late positivity has been associated with the recollective process by a number of authors. The amplitude of the later portion of the ERP waveform has been shown to increase when stimuli are presented for a second time (e.g., Bentin & Peled, 1990; Besson, Kutas, & Van Petten, 1992; Rugg, Furda, & Lorist, 1988). Segalowitz, Van Roon, and Dywan (1997) have demonstrated that further repetition of a word across a test session will result in continued increments in the late positivity. Similarly, increasing the degree of processing required at encoding (Paller, Kutas, & McIsaac, 1995) or increasing the complexity of the memory task (Wilding, Doyle, & Rugg, 1995) both lead to a greater amplitude late positivity in younger adults. On the basis of such data, the ERP late positivity has been associated with both familiarity and with the subjective experience of recollection. The higher the amplitude of the late positivity, the more it is assumed that the participant is engaged in a process of active recollection (e.g., Paller et al., 1995; Wilding et al., 1995; Wilding & Rugg, 1996). The lack of age differences in the old/new repetition effect demonstrated here and by others would suggest that older adults are as fully engaged as younger adults in whatever processes these tasks demand. However, the fact that older adults have been shown to produce greater amplitudes and longer duration late positivities during recognition is not entirely consistent with the view that greater amplitude reflects an increase in subjective levels of recollection. Even though age-related memory decrements are not as apparent in recognition paradigms as they are in free recall (e.g., Craik & McDowd, 1987; Rabinowitz, 1986), one would not expect indices of recollective experience to be greater in older relative to younger adults. In an attempt to reconcile this apparent inconsistency, the higher amplitude and increased duration late positivity in older adults has been interpreted as a sign of inefficient or additional processing (Friedman et al., 1993a; Karayanidis et al., 1993). This interpretation implies that there is a threshold with respect to the amplitude of an ERP response, whereby higher positive amplitude is a sign of higher level performance up to a point after which it is considered excess and a sign of impaired function. We will return to a discussion of this issue after considering the ERP responses during source monitoring. ERPS AND SOURCE MONITORING 423 Source Monitoring Effects The main purpose for this study was to determine whether measures of ERP responsivity during source monitoring would provide some indication of the neurophysiological processes associated with source error that occur prior to behavioral response. We had initially hypothesized, on the basis of the well-established similarity in the ERP repetition effect between older and younger adults, that the groups would experience familiarity for previously presented information to about the same degree. The experience of familiarity, according to this formulation, would involve a relatively automatic response to stimulus repetition. If so, younger adults would avoid source error by engaging consciously controlled processes, such as the specific recollection of context, in order to override this more automatic response to familiarity. Thus, for the younger adults, the experience of familiarity would be followed by explicit recollection of source. Older adults would simply be less likely to initiate the second process, i.e., they would be less likely to engage in the active processing necessary to make use of explicit recollection. However, our data suggest that stimulus classification, with respect to the old/new distinction, had occurred very early post stimulus onset so that by 350–400 ms a significant difference between study and lag items had begun to emerge. Thus, the controlled processing we attribute to the younger adults is unlikely to have been ‘‘tacked on’’ after a basic familiarity judgment had occurred. Rather, attentional processes involving expectancies and selectivity must have occurred in parallel with the initial perception of the event (see Mangun & Hillyard, 1990). Accounting for Source Error The fluency heuristic. Dissociations between ERP responsivity and target discriminability are not typically noted in standard old/new discrimination tasks. In the standard task there is no need to oppose familiarity in order to produce a correct response (e.g., Friedman et al., 1993b; Karayanidis et al., 1993; Rugg et al., 1994). However, it is the case that placing the familiarity and the ‘targetness’ of a stimulus in opposition allows one to observe dissociations between automatic and controlled processing as they relate to the accomplishment of task goals (e.g., Jennings & Jacoby, 1993; Jacoby, 1991). For older adults in this study, the ERP data indicate an undiminished responsivity to nontarget lag words. It is reasonable to speculate that more recently perceived words would be processed more fluently and, as such, give rise to the experience of familiarity (Jacoby, Kelley, & Dywan, 1989; Whittlesea, 1993). Johnston, Hawley, and Elliott (1991) report that perceptual fluency will contribute to incorrect ‘‘old’’ judgments when fluency is produced through perceptual memory and when explicit memory is minimal. We are, however, reluctant to assume a priori that the age effects in source 424 DYWAN, SEGALOWITZ, AND WEBSTER monitoring are due solely to a less articulated recollection of the study context on the part of the older adults. Age-related source error is not readily accounted for by age differences in the explicit recollection of content (Dywan & Jacoby, 1990; Dywan et al., 1994; Schacter et al., 1994). It has also been demonstrated that despite age differences in explicit memory for source, older adults benefit at least as much as younger adults when the perceptual characteristics of the stimuli are reinstated at test (Naveh-Benjamin & Craik, 1995). Moreover, reducing the degree to which perceptual fluency would be used as a basis for source monitoring decisions can eliminate age-related differences in source error (Multhaup, 1995; Masson, Carroll, & Micco, 1995). Thus, there is some evidence that older adults encode enough information to inform source judgements but that they are unlikely to use this information when self initiated, online monitoring is required or when the task allows dependence on a fluency heuristic. As well, we have demonstrated that young adults were as likely to make source error as older adults when distracted at test and that they produced the same reactivity to lag words in their ERP records as older adults. These data support the view that attentional capacity at test may be as important as encoding efficiency when one is faced with discriminating between sources of item familiarity. Reduced inhibitory control. The increased tendency on the part of older adults to make source monitoring errors could also be due to reduced inhibitory control over response tendencies. It has been argued that failed inhibitory control is central to age-related changes in cognitive function (e.g., Hasher & Zacks, 1988). Even though there is a growing body of data that can be interpreted within this framework (e.g., Zacks & Hasher, 1994), there are still questions as to the limits of inhibitory control as an explanatory model (e.g., Dywan & Murphy, 1996; Hartley & Hartley, 1996; Kramer, Humphrey, Larish, Logan, & Strayer, 1994). Moreover, there are a number of ways in which failed inhibitory control could have influenced performance in the present paradigm and it is not clear which of these might apply. First, it has been demonstrated that frontal lesions produce heightened responsivity in posterior sensory cortex (Knight, Scabini, & Woods, 1989; Yamaguchi & Knight, 1990). This heightened responsivity could account for age-related decrements in the ability to ignore irrelevant information (e.g., McDowd & Filion, 1992; Rabbit, 1965; Shimamura, et al., 1995). Second, there are data suggesting that reduced inhibitory control within the older CNS allows the excitation of neural circuits to persist longer. This extends the period during which neuronal circuits are refractory to incoming information and the effects of this are observed in a number of physiological systems (see Woodruff-Pak, 1988, for a review). A third dimension of inhibitory regulation involves the control of motor response tendencies (Drewe, 1975; Stuss, Kaplan, Benson, Weir, Chiulli, & Sarazin, 1982). Thus, to the degree that frontally based neural processes are rendered less efficient with age (e.g., Martin, Friston, Colebatch, & Frackowiak, 1991), ERPS AND SOURCE MONITORING 425 the older adult would not only have to cope with higher levels of posterior cortex sensory reactivity but this reactivity would reverberate through the system longer, perhaps accounting for the longer duration late positivities. In this experimental paradigm, these changes, combined with reduced control of motor response tendencies, could leave an older adult at greater risk for source error even if the memory trace were sufficient to support a correct response. Friedman and Simpson (1994), using a simple target detection task with no appreciable memory component, report that older adults are more likely than younger adults to make false positive responses to unique and unexpected novel stimuli. The ERP response to these stimuli did not show the same degree of habituation across trials that had been observed for the younger and middle-aged adults. Observing the performance of our subjects, it was obvious that the older adults and the young adults in the dual-task condition often realized that they had made a lag error as they were in the process of hitting the wrong response key. Their frustration at their own tendency to make lag errors coupled with the excess neural reactivity noted in their ERP response to the lag words, whether correctly of incorrectly classified, would suggest that the source memory problems noted in this sample of older adults could not be fully explained by weaker memory representations although further study is required to determine this with certainty. While the effects of dual-task conditions on our young adults would suggest that attentional capacity is highly relevant with respect to inhibitory control, further study will be required to more precisely determine the mechanisms responsible for the deficits in inhibitory control. Possible explanations include a hyperreactivity to the initial perceptual processing of the sensory stimulus (e.g., Knight et al., 1989), a delayed refractory period that maintains stimulation longer in the older CNS (e.g., Woodruff-Pak, 1988), or the ability to inhibit a motor response (Stuss et al., 1982). CONCLUSIONS We conclude that older adults are more likely to make source errors than younger adults because a reduced attentional capacity leads to higher levels of neural responsivity to repeated information. In this study, the repeat of a lag word occurred after a shorter time delay than the repeat of a study word. This may have enhanced the salience of lag words relative to study words within the experimental paradigm and thus increased the likelihood of a false positive response. Of course, there may be other factors that could increase the salience of distracting information and, as a consequence, the tendency to make source error (e.g., Hashtroudi et al., 1990; Jacoby et al., 1989; Whittlesea, 1993). What these data suggest is that the ERP late positivity effect may not necessarily be a direct index of familiarity, recognition, or recollection. It 426 DYWAN, SEGALOWITZ, AND WEBSTER may, instead, represent the degree to which a stimulus appears salient to the individual. What appears salient may be differentially determined as a function of the task the subject is asked to do (e.g., Johnson, Kounios, & Nolde, 1997; Osterhout et al., 1996; Wilding & Rugg, 1996) and the age of the person performing it (Johnson, 1995; Senkfor & Van Petten, 1995; Swick & Knight, 1997). We propose that the reactivity of the younger nervous system, under normal conditions, is much less influenced by stimulus properties, such as the recency of item repetition, and much more by task goals which suggests more active attentional allocation early in the response process. From this perspective, the ERP late positivity would represent a general neural reactivity to salient events which would, as attentional resources wane, be as likely to mislead as inform mnemonic decisions. REFERENCES Bartlett, J. C., Strater, L., & Fulton, A. 1991. False recency and false fame of faces in young adulthood and old age. Memory and Cognition, 19, 177–188. Bentin, S., & Peled, B. S. 1990. The contribution of stimulus encoding strategies and decisionrelated factors to the repetition effect for words: Electrophysiological evidence. Memory and Cognition, 18, 358–366. Bentin, S., & McCarthy, G. 1994. The effects of immediate stimulus repetition on reaction time and event-related potentials in tasks of different complexity. Journal of Experimental Psychology, 20, 130–149. Besson, M., Kutas, M., & Van Petten, C. 1992. An event-related potential (ERP) analysis of semantic congruity and repetition effects in sentences. Journal of Cognitive Neuroscience, 4, 132–149. Chalfonte, B. L., & Johnson, M. K. 1996. Feature memory and binding in young and older adults. Memory and Cognition, 24, 403–416. Cohen, G., & Faulkner, D. 1989. Age differences in source forgetting: Effects on reality monitoring and on eyewitness testimony. Psychology and Aging, 4, 10–17. Craik, F. I. M., & McDowd, J. M. 1987. Age differences in recall and recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition, 13, 474–479. Craik, F. I. M., Morris, L. W., Morris, R. G., & Loewen, E. R. 1990. Relations between source amnesia and frontal lobe functioning in older adults. Psychology and Aging, 5, 148–151. Diamond, A. 1990. Developmental time course in human infants and infant monkeys, and the neural bases, of inhibitory control in reaching. Annals of the New York Academy of Sciences, 608, 637–676. Drewe, E. A. 1975. Go no-go learning after frontal lobe lesions in humans. Cortex, 11, 8– 16. Dywan, J., & Jacoby, L. L. 1990. Effects of aging on source monitoring: Differences in susceptibility to false fame. Psychology and Aging, 5, 379–387. Dywan, J., & Murphy, W. E. 1996. Aging and inhibitory control in text comprehension. Psychology and Aging, 11, 199–206. Dywan, J., & Segalowitz, S. J. 1996. Self- and family ratings of adaptive behavior after traumatic brain injury: Psychometric scores and frontally generated ERPs. The Journal of Head Trauma Rehabilitation, 11, 79–95. Dywan, J., Segalowitz, S. J., & Williamson, L. 1994. Source monitoring during name recogni- ERPS AND SOURCE MONITORING 427 tion in older adults: Psychometric and electrophysiological correlates. Psychology and Aging, 9, 568–577. Engle, R. W., Conway, A. R. A., Tuholski, S. W., & Shisler, R. J. 1995. A resource account of inhibition. Psychological Science, 6, 122–125. Fabiani, M., Karis, D., & Donchin, E. 1986. P300 and recall in an incidental memory paradigm. Psychophysiology, 23, 298–308. Friedman, D. 1990. ERPs during continuous recognition memory for words. Biological Psychology, 30, 61–88. Friedman, D., Berman, S., & Hamberger, M. 1993a. Recognition memory and ERPs: Agerelated changes in young, middle-aged, and elderly adults. Journal of Psychophysiology, 7, 181–201. Friedman, D., Hamberger, M., & Ritter, W. 1993b. Event-related potentials as indicators of repetition priming in young and older adults: Amplitude, duration, and scalp distribution. Psychology and Aging, 8, 120–125. Friedman, D., Hamberger, M., Stern, Y., and Marder, K. 1992. Event-related potentials (ERPs) during repetition priming in Alzheimer’s patients and young and older controls. Journal of Clinical and Experimental Neuropsychology, 14, 448–462. Friedman, D., & Simpson, G. V. 1994. ERP amplitude and scalp distribution to target and novel events: Effects of temporal order in young, middle-aged and older adults. Cognitive Brain Research, 2, 49–63. Fuster, J. M. 1990. Inferotemporal units in selective visual attention and short-term memory. Journal of Neurophysiology, 64, 681–697. Godefroy, O., & Rousseaux, M. 1996. Divided and focused attention in patients with lesion of the prefrontal cortex. Brain and Cognition, 30, 155–174. Goldman-Rakic, P. S. 1988. Topography of cognition: Parallel distributed networks in primate association cortex. Annual Review of Neurosciences, 11, 137–156. Grafman, J. 1994. Alternative frameworks for the conceptualization of prefrontal lobe functions. In F. Boller & J. Grafman (Eds.), Handbook of neuropsychology, Vol. 9 (pp. 187– 202). New York: Elsevier. Gruppuso, V., Lindsay, D. S., Kelley, C. M. 1997. The process-dissociation procedure and similarity: Defining and estimating recollection and familiarity in recognition memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 23, 259–278. Hamberger, M., & Friedman, D. 1992. Event-related potential correlates of repetition priming and stimulus classification in young, middle-aged, and older adults. Journals of Gerontology: Psychological Sciences, 47, P395–P405. Hartley, J., & Hartley, A. April, 1996. Putting a stop to assertions that older adults have difficulty inhibiting a response. Paper presented at the Cognitive Aging Conference, Atlanta, GA. Hasher, L., & Zacks, R. T. 1988. Working memory, comprehension, and aging: A review and a new view. The Psychology of Learning and Motivation, 99, 122–149. Hashtroudi, S., Johnson, M. K., & Chrosniak, L. D. 1989. Aging and source monitoring. Psychology and Aging, 4, 106–112. Hashtroudi, S., Johnson, M. K., & Chrosniak, L. D. 1990. Aging and qualitative characteristics of memories for perceived and imagined complex events. Psychology and Aging, 5, 119– 126. Heaton, R. K. 1981. A manual for the Wisconsin Card Sorting Test. Odessa, FL: Psychological Assessment Resources. Jacoby, L. L. 1991. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language, 30, 513–541. 428 DYWAN, SEGALOWITZ, AND WEBSTER Jacoby, L. L., Kelley, C. M., & Dywan, J. 1989. Memory attributions. In H. L. Roediger & F. I. M. Craik (Eds.), Varieties of memory and consciousness: Essays in honour of Endel Tulving pp. 391–422). Hillsdale, NJ: Erlbaum. Jacoby, L. L., Toth, J. P., & Yonelinas, A. P. 1993. Separating conscious and unconscious influences of memory: Measuring recollection. Journal of Experimental Psychology: General, 122, 139–154. Jacoby, L. L., Woloshyn, V., & Kelley, C. M. 1989. Becoming famous without being recognized: Unconscious influences of memory produced by dividing attention. Journal of Experimental Psychology: General, 118, 115–125. Jennings, J. M., & Jacoby, L. L. 1993. Automatic versus intentional uses of memory: Aging, attention, and control. Psychology and Aging, 8, 283–293. Jennings, J. M., & Jacoby, L. L. 1997. An opposition procedure for detecting age-related deficits in recollection: Telling effects of repetition. Psychology and Aging, 12, 352–361. Johnson, M. K., & Chalfonte, B. L. 1994. Binding complex memories: The role of reactivation and the hippocampus. In D. L. Schacter & E. Tulving (Eds.), Memory systems 1994 (pp. 311–350). Cambridge, MA: MIT Press. Johnson, M. K., De Leonardis, D. M., Hashtroudi, S., & Ferguson, S. A. 1995. Aging and single versus multiple cues in source monitoring. Psychology and Aging, 10, 507–517. Johnson, M. K., Hashtroudi, S., & Lindsay, D. S. 1993. Source monitoring. Psychological Bulletin, 114, 3–28. Johnson, M. K., Kounios, J., & Nolde, S. F. 1997. Electrophysiological brain activity and memory source monitoring. Neuro Report, 8, 1317–1320. Johnson, R. 1995. Event-related potential insights into the neurobiology of memory systems. In F. Boller & J. Grafman (Eds.), Handbook of neuropsychology, Vol. 10 (pp. 135– 163). New York: Elsevier. Johnston, W. A., Hawley, K. J., & Elliott, J. M. G. 1991. Contribution of perceptual fluency to recognition judgments. Journal of Experimental Psychology: Learning, Memory, and Cognition, 17, 210–223. Karayanidis, F., Andrews, S., Ward, P. B., & McConaghy, N. 1993 Event-related potentials and repetition priming in young, middle-aged and elderly normal subjects. Cognitive Brain Research, 1, 123–134. Kausler, D. H., & Wiley, J. G. 1990. Temporal memory and content memory for actions: Adult age differences in acquisition and retention. Experimental Aging Research, 16, 147–150. Knight, R. T., Scabini, D., & Woods, D. L. 1989. Prefrontal cortex gating of auditory transmission in humans. Brain Research, 504, 338–342. Kramer, A. F., Humphrey, D. G., Larish, J. F., Logan, G. D., & Strayer, D. L. 1994. Aging and inhibition: Beyond a unitary view of inhibitory processes in attention. Psychology and Aging, 9, 491–512. Kučera, H., & Francis, W. 1967. Computational analysis of present-day American English. Providence, RI: Brown. Lehman, E. B., & Mellinger, J. C. 1984. Effects of aging on memory for presentation modality. Developmental Psychology, 20, 1210–1217. Light, L. L., La Voie, D., Valencia-Laver, D., Albertson-Owens, S. A., & Mead, G. 1992. Direct and indirect measures of memory for modality in young and older adults. Journal of Experimental Psychology: Learning, Memory and Cognition, 18, 1284–1297. Malloy, P., & Duffy, J. 1994. The frontal lobes and neuropsychiatric disorders. In F. Boller & J. Grafman (Eds.), Handbook of neuropsychology, Vol. 9 (p. 203). New York: Elsevier. Mangun, G. R., & Hillyard, S. A. 1990. Electrophysiological studies of visual selective atten- ERPS AND SOURCE MONITORING 429 tion in humans. In A. Scheibel & A. Wechsler (Eds.), The neurobiological foundations of higher cognitive function (pp. 271–294). New York: Guilford. Martin, A. J., Friston, K. J., Colebatch, J. G., & Frackowiak, R. S. J. 1991. Decreases in regional cerebral blood flow with normal aging. Journal of Cerebral Blood Flow Metabolism, 11, 694–689. Masson, M. E. J., Carroll, M., & Micco, A. 1995. Attributions of fluency in fame judgments by younger and older adults. Canadian Journal of Experimental Psychology, 49, 287– 310. McCarthy, G., & Wood, C. C. 1985. Scalp distributions of event-related potentials: An ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology, 62, 203–208. McDowd, J., & Filion, D. 1992. Aging, selective attention, and inhibitory processes: A psychophysiological approach. Psychology and Aging, 7, 65–71. Moscovitch, M. 1994. Memory and working with memory: Evaluation of a component process model and comparisons with other models. In D. L. Schacter & E. Tulving (Eds.), Memory systems 1994 (pp. 269–310). Cambridge, MA: MIT Press. Multhaup, K. S. 1995. Aging, source, and decision criteria: When false fame errors do and do not occur. Psychology and Aging, 10, 492–497. Naveh-Benjamin, M., & Craik, F. I. M. 1995. Memory for context and its use in item memory: Comparisons of younger and older persons. Psychology and Aging, 10, 284–293. Osterhout, L., McKinnon, R., Bersick, M., & Corey, V. 1996. On the language specificity of the brain response to syntactic anomalies: Is the syntactic positive shift a member of the P300 family? Journal of Cognitive Neuroscience, 8, 507–526. Paller, K. A., & Kutas, M. 1992. Brain potentials during memory retrieval provide neurophysiological support for the distinction between conscious recollection and priming. Journal of Cognitive Neuroscience, 4, 375–391. Paller, K. A., Kutas, M., & McIsaac, H. K. 1995. Monitoring conscious recollection via the electrical activity of the brain. Psychological Science, 6, 107–111. Petrides, M. 1994. Frontal lobes and working memory: Evidence from investigations of the effects of cortical excisions in nonhuman primates. In F. Boller & J. Grafman (Eds.), Handbook of neuropsychology, Vol. 9 (pp. 59–82). New York: Elsevier. Pribram, K. H., & McGuinness, D. 1992. Attention and para-attentional processing: Eventrelated brain potentials as tests of a model. Annals of the New York Academy of Sciences, 68, 65–92. Puglisi, J. T., Park, D. C., Smith, A. D., & Hill, G. W. 1985. Memory for two types of spatial location: Effects of instructions, age, and format. American Journal of Psychology, 98, 101–118. Rabbit, P. 1965. An age decrement in the ability to ignore irrelevant information. Journal of Gerontology, 20, 233–236. Rabinowitz, J. C. 1986. Priming in episodic memory. Journal of Gerontology, 41, 204–213. Rosahl, S. K., & Knight, R. T. 1995. Role of prefrontal cortex in generation of the contingent negative variation. Cerebral Cortex, 5, 123–134. Rugg, M. D. 1985. The effects of word repetition and semantic priming on event-related potentials. Psychophysiology, 22, 642–647. Rugg, M. D., Furda, J., & Lorist, M. 1988. The effects of task on the modulation of eventrelated potentials by word repetition. Psychophysiology, 25, 55–63. Rugg, M. D., Pearl, S., Walker, P., Roberts, R. C., & Holdstock, J. S. 1994. Word repetition effects on event-related potentials in healthy young and old subjects, and in patients with Alzheimer-type dementia. Neuropsychologia, 32, 381–398. 430 DYWAN, SEGALOWITZ, AND WEBSTER Salthouse, T. A. 1991. Mediation of adult age differences in cognition by reductions in working memory and speed of processing. Psychological Science, 2, 179–183. Schacter, D. L., Osowiecki, Kaszniak, A. W., Kihlstrom, J. F., & Valdiserri, M. 1994. Source memory: Extending the boundaries of age-related defects. Psychology and Aging, 9, 81– 89. Schacter, D. L., & Tulving, E. 1994. What are the memory systems of 1994? In D. L. Schacter & E. Tulving (Eds.), Memory systems 1994 (pp. 1–38). Cambridge, MA: MIT Press. Segalowitz, S. J., Van Roon, P., & Dywan, J. 1997. The ERP late positivity: A graduated response to stimulus repetition. NeuroReport, 8, 757–760. Senkfor, A. J., & Van Petten, C. October, 1995. Who said what? ERP measures of source and item memory. Paper presented at the Society for Psychophysiological Research, Toronto, Ontario. Shimamura, A. P. 1994. Frontal lobes and memory. In M. S. Gazzaniga (Ed.), The cognitive neurosciences (pp. 803–813). Cambridge, MA: MIT Press. Shimamura, A. P., Jurica, P. J., Mangels, J. A., Gershberg, F. B., & Knight, R. T. 1995. Susceptibility to memory interference effects following frontal lobe damage: Findings from tests of paired-associate learning. Journal of Cognitive Neuroscience, 7, 144–152. Shimamura, A. P., & Squire, L. R. 1987. A neuropsychological study of fact memory and source amnesia. Journal of Experimental Psychology: Learning, Memory, and Cognition, 13, 464–473. Smith, M. E. 1993. Neurophysiological manifestations of recollective experience during recognition memory judgements. Journal of Cognitive Neurosciences, 5, 1–13. Spencer, W. D., & Raz, N. 1994. Memory for facts, source, and context: Can frontal lobe dysfunction explain age-related differences? Psychology and Aging, 9, 149–159. Spencer, W. D., & Raz, N. 1995. Differential effects of aging on memory for content and context: A meta-analysis. Psychology and Aging, 10, 527–539. Stuss, D. T., Kaplan, E. F., Benson, D. F., Weir, W. S., Chiulli, S., & Sarazin, F. F. 1982. Evidence for the involvement of orbitofrontal cortex in memory functions: An interference effect. Journal of Comparative Physiological Psychology, 96, 913–925. Swick, D., & Knight, R. T. 1997. Event-related potentials differentiate the effects of aging on word and nonword repetition in explicit and implicit memory tasks. Journal of Experimental Psychology: Learning, Memory, & Cognition, 23, 123–142. Verleger, R., Gasser, T., & Mocks, J. 1982. Correction of EOG artifacts in event-related potentials of the EEG: Aspects of reliability and validity. Psychophysiology, 19, 472–480. Whittlesea, B. W. A. 1993. Illusions of familiarity. Journal of Experimental Psychology: Learning, Memory, and Cognition, 19, 1235–1253. Wilding, E. L., Doyle, M. C., & Rugg, M. D. 1995. Recognition memory with and without retrieval or context: An event-related potential study. Neuropsychologia, 33, 743–767. Wilding, E. L., & Rugg, M. D. 1996. An event-related potential study of recognition memory with and without retrieval of source. Brain, 119, 889–905. Woodruff-Pak, D. S. 1988. Psychology and aging. Englewood Cliffs, NJ: Prentice Hall. Yamaguchi, S., & Knight, R. T. 1990. Gating of somatosensory input by human prefrontal cortex. Brain Research, 521, 281–288. Zacks, R. T., & Hasher, L. 1994. Directed ignoring: Inhibitory regulation of working memory. In D. Dagenbach & T. H. Carr (Eds.), Inhibitory processes in attention, memory, and language (pp. 241–264). San Diego: Academic Press. Zelinski, E. M., & Light, L. L. 1988. Young and older adults’ use of context in spatial memory. Psychology and Aging, 3, 99–101.