Unit 7: Alkane ppt - Solon City Schools
advertisement

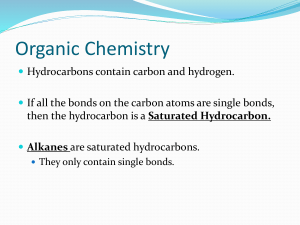
Organic Chemistry Hydrocarbons contain carbon and hydrogen. If all the bonds on the carbon atoms are single bonds, then the hydrocarbon is a Saturated Hydrocarbon. Alkanes are saturated hydrocarbons. They only contain single bonds. Alkanes Have the general formula CnH2n+2 (where n= the number of carbon atoms). When naming them, the name ends in –ane. All the carbons are bonded to 4 other atoms. Name Methane Ethane Molecular Formula CnH2n+2 Structural Formula Model Propane Butane 2-Methylpropane (an isomer of butane) Example 1: Draw: 3-ethylheptane Example 2: Draw: 2,7-dimethylnonane Example 3: Draw: 4-ethyl-2,4,5-trimethyloctane Example 4: 3,3,4,4-tetraethyl-2,2,5,5-tetramethylhexane Cycloalkanes All single bonds between the carbon atoms. Have the general formula CnH2n The ends of the carbon chain have bonded together to form a “ring” of carbons (lose 2 hydrogen atoms when this happens). Examples: Draw: cyclobutane 1,3-dimethylcylcopentane 1-ethyl-3-methyl-2-propylcyclobutane Rules for naming: 1) Find the parent chain (the longest continuous chain of carbon atoms); helpful to circle or put a box around the parent chain. 2) Number the carbon atoms on the parent chain so that the branches get the lowest combination of numbers; you can number it left to right or right to left. 3) Name the branches with –yl ending; if there are multiple of the same branch, use a prefix with the branch (di-, tri-, tetra-, penta-, hexa-, hepta-, octa-) 4) Put the branches with their # in alphabetical order. 5) The parent chain name goes at the end and ends in –ane.