Ectomycorrhizal fungi: exploring the mycelial frontier
Ian C. Anderson1 & John W.G. Cairney2
1
The Macaulay Institute, Craigiebuckler, Aberdeen, UK; and 2Centre for Plant and Food Science, University of Western Sydney, NSW, Australia
Correspondence: Ian C. Anderson, The
Macaulay Institute, Craigiebuckler, Aberdeen
AB15 8QH, UK. Tel.: 144 1224 498200;
ext. 2357; fax: 144 1224 498207; e-mail:
i.anderson@macaulay.ac.uk
Received 1 December 2006; revised 12
February 2007; accepted 5 March 2007.
DOI:10.1111/j.1574-6976.2007.00073.x
Editor: Ramon Diaz Orejas
Keywords
ectomycorrhizal fungi; soil-borne mycelia;
mycelial biomass; ectomycorrhizal
communities; elevated atmospheric CO2.
Abstract
Ectomycorrhizal (ECM) fungi form mutualistic symbioses with many tree species
and are regarded as key organisms in nutrient and carbon cycles in forest
ecosystems. Our appreciation of their roles in these processes is hampered by a
lack of understanding of their soil-borne mycelial systems. These mycelia represent
the vegetative thalli of ECM fungi that link carbon-yielding tree roots with soil
nutrients, yet we remain largely ignorant of their distribution, dynamics and
activities in forest soils. In this review we consider information derived from
investigations of fruiting bodies, ECM root tips and laboratory-based microcosm
studies, and conclude that these provide only limited insights into soil-borne ECM
mycelial communities. Recent advances in understanding soil-borne mycelia of
ECM fungi have arisen from the combined use of molecular technologies and
novel field experimentation. These approaches have the potential to provide
unprecedented insights into the functioning of ECM mycelia at the ecosystem
level, particularly in the context of land-use changes and global climate change.
Introduction
Many tree species in forest habitats worldwide rely upon
mutualistic ectomycorrhizal (ECM) fungi to fulfil their
nutrient requirements (Smith & Read, 1997). These fungi
contribute to tree nutrition by means of mineral weathering
(Landeweert et al., 2001) and mobilization of nutrients from
organic complexes (Read & Perez-Moreno, 2003). They are
also an important avenue for the delivery of carbon to soil
and are responsible for a substantial component of forestsoil carbon fluxes (Söderström, 1992; Högberg et al., 2001;
Högberg & Högberg, 2002; Godbold et al., 2006; Hobbie,
2006). ECM fungi are thus regarded as key elements of forest
nutrient cycles and as strong drivers of forest ecosystem
processes (Read et al., 2004).
The fungi that form ECM associations comprise a taxonomically broad suite of basidiomycetes and, to a lesser
extent, ascomycetes (Smith & Read, 1997). Typically, the
fungi form a mycelial mantle around short lateral roots of
their hosts and penetrate between epidermal and cortical
cells, surrounding them with a highly branched structure,
the Hartig net (Peterson et al., 2004). Significant variation
exists in the morphology of ECM root tips that are infected
by different fungal taxa, and analysis of macroscopic and
microscopic characteristics of ECM roots is used widely for
identification of the ECM fungi (Agerer, 1987–2002). The
fungus–plant interface formed by the Hartig net in the ECM
root is functionally critical to the mutualism, as it represents
FEMS Microbiol Rev ]] (2007) 1–19
the interface across which nutrients and carbon are transferred between the partners (Smith & Read, 1997).
In addition to the structures formed at the host root,
ECM fungi produce mycelia that extend from the mantle
into the surrounding soil (Fig. 1), although the extent and
structure of this extramatrical mycelium is thought to differ
between ECM fungal taxa (Agerer, 2001). The soil-borne
mycelia of ECM fungi are regarded as functionally important to the mutualism in foraging for, and translocation of,
nutrients and water. They also infect short lateral roots
(Smith & Read, 1997) and potentially form a common
mycelial network that might facilitate carbon and/or nutrient movement between individual tree hosts (Simard &
Durall, 2004; Selosse et al., 2006). At the ecosystem level
these mycelia are of further significance in the mobilization
of nutrients from organic and recalcitrant inorganic sources,
both in competition and in cooperation with soil-dwelling
saprotrophs, in the relocation of nutrients within the
soil–plant continuum, and in the delivery and distribution
of carbon belowground (Leake et al., 2002; Wallander,
2006). Despite the importance of soil-borne mycelia of
ECM fungi in forest ecosystems, along with their likely
importance in the global carbon budget (Hobbie, 2006)
and sensitivity to global change factors such as rising atmospheric CO2 (Rillig et al., 2002), we know relatively little
regarding their extent, diversity and spatial location in forest
soils. In particular, the current lack of fundamental
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
2
information regarding the physical and physiological continuity of belowground mycelia limits our understanding of
the importance of ECM mycelial networks in nutrient
cycling and in the distribution of carbon and nutrients
within forest-soil systems (Cairney, 2005). Furthermore,
although many detailed investigations of communities of
ECM fungi colonizing root tips have been undertaken (see
below), the relative importance of particular taxa in root-tip
communities gives no insight into the extent of their
I.C. Anderson et al.
mycelial systems in soil (Genney et al., 2006), nor into their
contributions to nutrient and carbon cycling.
To date, investigation of ECM mycelia in soil has been
constrained by the difficulty in identifying ECM mycelia and
in separating these from mycelia of non-ECM soil fungi.
Most of our current understanding has thus been derived via
inference from the distribution of fruiting bodies and ECM
root tips in the field and/or from observations of mycelia
produced in microcosms in the laboratory (Fig. 1). As we
Fig. 1. Mycelial systems of ectomycorrhizal fungi. (a) A typical microcosm system demonstrating the mycelium of Suillus variegatus growing from a
Scots pine seedling (photo taken by P.M.A. Fransson). (b) Russula sp. fruiting body and associated mycelium. (c) A close-up of (b) showing the base of
the fruiting-body stipe, the associated white mycelium, and ECM root tips (arrows) colonized by this fungus. (d) A dense mycelial mat that has been
lifted from a rock surface in a native Scots pine forest. Patches of brown, pink, white and yellow mycelium of different fungal species are visible (photo
taken by C.D. Campbell).
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
FEMS Microbiol Rev ]] (2007) 1–19
3
Ectomycorrhizal fungal mycelia
will outline in this review, many of the assumptions upon
which these inferences are based are somewhat tenuous, and
current understanding of complex ECM mycelial systems in
forest soils remains limited. Recent years have, however,
witnessed the development and application of a suite of
molecular methods that are yielding unprecedented insights
into ECM mycelia in forest soils. In this review we highlight
some of the limitations of our current knowledge, along
with the most significant recent advances in our appreciation of the distribution, dynamics and activities of soilborne ECM mycelial systems along with their responses to
elevated atmospheric CO2 concentrations.
Fruiting bodies as indicators of
community structure
Although frequently used in surveys of higher fungal
diversity, fruiting bodies provide little useful information
regarding the diversity of ECM fungal mycelia in soil.
Clearly, the presence of an epigeous fruiting body indicates
that mycelium of the fungus must be present in one form or
another in the underlying soil. Because many ECM taxa
produce no epigeous fruiting bodies, or may not have
fruited during the duration of the survey, it is impossible to
infer the importance of a fungus in the belowground
community on the basis of fruiting-body observations. In
fact, where fruiting-body data have been compared with
ECM root tips (see below), there is generally a very poor
correlation between the two, with the latter typically indicating greater species richness (Erland & Taylor, 2002).
Furthermore, there is evidence that, for Laccaria spp. at
least, physiological and/or genetic differences may influence
fruiting at the intraspecific level (Selosse et al., 2001).
Estimation of mycelial distribution using
fruiting bodies
For those ECM fungi that produce epigeous fruiting bodies,
their presence has been used to infer the distribution of
mycelia of the fungi in forest soils. This method centres on
analysis of the mycelial genotype in the vegetative tissue of
the fruiting body and mapping the distribution of the
genotypes in a forest stand. Fruiting bodies that have the
same vegetative genotype must have developed from a
genetically identical mycelium that has grown through soil,
thus allowing estimation of the distribution of soil-borne
mycelia (Dahlberg & Stenlid, 1995). Initially this was
achieved by isolation of vegetative fruiting-body stipe mycelium into axenic culture, followed by somatic incompatibility testing to identify which isolates are genetically
identical (e.g. Dahlberg & Stenlid, 1990, 1994; Baar et al.,
1994). While useful for fungi that are readily cultured, many
ECM fungi are difficult or impossible to isolate into axenic
FEMS Microbiol Rev ]] (2007) 1–19
culture by conventional methods (Brundrett et al., 1996),
limiting the usefulness of somatic compatibility testing for
ECM fungi. Concerns have also been raised about the
resolution of the method for identifying genotypes of some
ECM taxa (Sen, 1990; Jacobson et al., 1993).
More recently, molecular methods for the identification
of genotypes using DNA extracted from vegetative fruitingbody tissue have been used in this context. A range of
dominant molecular markers, including random amplified
polymorphic DNA (RAPD), simple sequence repeat (SSR),
amplified fragment length polymorphism (AFLP), singlestrand conformational polymorphism (SSCP) and interretrotransposon amplified polymorphism (IRAP) markers
(e.g. De la Bastide et al., 1994; Anderson et al., 1998; Bonello
et al., 1998; Sawyer et al., 1999; Redecker et al., 2001; Murata
et al., 2005), along with codominant repeat SSR markers
(e.g. Zhou et al., 2001a; Dunham et al., 2003; Kretzer et al.,
2004; Wu et al., 2005; Bergemann et al., 2006), has now been
used to infer the genotype distributions of certain ECM
fungi in a variety of forest habitats. While some of these
molecular methods may not resolve all genotypes in a
population (Redecker et al., 2001; Dunham et al.,
2003; Bagley & Orlovich, 2004), they have been widely
used to estimate the spatial distribution of ECM fungal
genotypes.
It is thus evident that genotypes of some ECM fungi are
spread over tens of metres in some forests, implying that
mycelia can extend for considerable distances, and persist
for several years, in the field (e.g. Dahlberg & Stenlid, 1990;
Baar et al., 1994; Dahlberg, 1997; Anderson et al., 1998;
Bonello et al., 1998; Sawyer et al., 1999). Estimates of
belowground mycelial growth rates (e.g. Dahlberg & Stenlid,
1990; Bonello et al., 1998; Sawyer et al., 1999; Lian et al.,
2006) and investigations of the survival of genotypes introduced as ECM inoculum in forest plantations (e.g. De la
Bastide et al., 1994; Selosse et al., 1998; Sawyer et al., 2001)
provide strong supplementary evidence that mycelia of
certain ECM fungi can persist in forest soils for at least tens
of years. Whether these genotypes persist in soil as continuous mycelia [ = genets (Dahlberg & Stenlid, 1995)], or have
fragmented into smaller mycelia [ = ramets (Dahlberg &
Stenlid, 1995)] as a result of, for example, feeding activities
of mycophagous soil arthropods (Schneider et al., 2005) or
other forms of disturbance, remains unclear.
Fruiting-body collections indicate that multiple genotypes of some species, such as Laccaria spp., frequently
occur in an area of a few square metres, suggesting the
presence of many spatially restricted and ephemeral mycelia
in the underlying soil (e.g. Gryta et al., 1997; Gherbi et al.,
1999; Fiore-Donno & Martin, 2001; Dunham et al., 2003;
Murata et al., 2005). Within genera such as Amanita
(Redecker et al., 2001; Sawyer et al., 2001, 2003; Bagley &
Orlovich, 2004; Liang et al., 2005), Russula (Redecker et al.,
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
4
2001; Bergemann & Miller, 2002; Liang et al., 2004; Riviere
et al., 2006) or Tricholoma (Murata et al., 2005; Gryta et al.,
2006; Lian et al., 2006) there are varying reports of widespread and spatially restricted genotypes. Similar observations have been made in population studies of individual
species such as Suillus bovinus (Dahlberg & Stenlid, 1990,
1994), Laccaria bicolor (Baar et al., 1994; Selosse et al., 1998)
and Pisolithus albus (Anderson et al., 1998, 2001), making it
difficult to predict genotype distribution, and so infer the
dimensions of belowground mycelia, strictly on the basis of
taxonomic affiliation.
The occurrence of multiple spatially restricted genotypes
of an ECM fungus is thought to reflect frequent mycelial
establishment from meiospores, while a local population
dominated by widespread genotypes is characteristic of the
sustained growth of persistent belowground mycelia (Dahlberg & Stenlid, 1995). There is some evidence that, for
certain ECM fungi, the former may prevail in recently
disturbed or younger forests, while the latter predominate
in mature undisturbed forests (Dahlberg & Stenlid, 1990,
1995). This is not always the case, however, because the
distribution of host tree species in a mixed forest may
influence the distribution of ECM genotypes (Zhou et al.,
2000). Furthermore, multiple spatially restricted genotypes
have been identified in populations of some ECM fungi in
mature forests (e.g. Gherbi et al., 1999; Fiore-Donno &
Martin, 2001; Redecker et al., 2001), and widespread genotypes in relatively young plantations (Selosse, 2003). The
meaning of these observations in terms of the distribution of
soil-borne mycelia is questionable. At best, such information
based on fruiting-body distribution allows estimation of
possible mycelial dimensions in the underlying soil. It
provides no information on mycelial continuity, density or
vertical distribution in the soil profile.
Community structure and spatial
distribution based on ectomycorrhizal
root tips
Root tip-based assessment of belowground ECM
fungal diversity
With the realization that analysis of ECM fungal communities on the basis of fruiting-body data is a poor indicator
of belowground communities of the fungi (Horton & Bruns,
2001; Erland & Taylor, 2002) came acceptance that communities of ECM fungi are best analysed on the basis of
identification and enumeration of short lateral roots infected by ECM fungal taxa ( = ECM roots). Initially achieved
by morphological identification of ECM roots (e.g. Taylor &
Alexander, 1989; Massicotte et al., 1999), PCR-based molecular approaches have become de rigueur for these investi2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
I.C. Anderson et al.
gations in recent years (Horton & Bruns, 2001; Anderson,
2006), and a great deal of thought has been invested in
designing appropriate sampling strategies (e.g. Horton &
Bruns, 2001; Taylor, 2002). Although some specific habitats
appear to be characterized by low species diversity of ECM
roots (Chambers et al., 2005), numerous such investigations
have revealed that communities of ECM roots in multifarious forest habitats are generally extremely species-rich
(reviewed Horton & Bruns, 2001). It is also evident that
communities of ECM roots vary with vegetation/environmental gradients and chronosequences (e.g. Kernaghan &
Harper, 2001; Wurzenburger et al., 2004; Dickie & Reich,
2005; Palfner et al., 2005; Robertson et al., 2006).
ECM root species richness and/or community structure
are influenced by a range of factors, including soil type
(Gehring et al., 1998; Moser et al., 2005), soil moisture (e.g.
Taylor & Alexander, 1989; Kårén et al., 1996; Shi et al., 2002;
Walker et al., 2005), season (Giachini et al., 2004), natural
nutrient gradients (Toljander et al., 2006), fire (e.g. Visser,
1995; Stendell et al., 1999; Grogan et al., 2000; Smith et al.,
2005; and see the review by Cairney & Bastias, 2006),
activities of herbivores and plant parasites (Brown et al.,
2001; Cullings et al., 2005; Mueller & Gehring, 2006), and
the quality and quantity of organic matter (e.g. Conn &
Dighton, 2000; Cullings et al., 2003). ECM root-tip communities can also be strongly influenced by a range of forest
management practices (reviewed Jones et al., 2003) and by
gradients of nitrogen deposition (Taylor et al., 2000; Lilleskov et al., 2002; Dighton et al., 2004). Further anthropogenic
factors that have been shown to influence ECM root communities include localized nitrogen addition (e.g. Kårén &
Nylund, 1997; Fransson et al., 2000; Peter et al., 2001; Avis
et al., 2003; Berch et al., 2006; Carfrae et al., 2006), acid
precipitation (e.g. Rapp & Jentschke, 1994; Qian et al., 1998;
Roth & Fahey, 1998), elevated atmospheric CO2 concentrations (Rey & Jarvis, 1997; Fransson et al., 2001; Kasurinen
et al., 2005), toxic metal contamination (e.g. Markkola et al.,
2002), and other forms of anthropogenic pollution (reviewed Cairney & Meharg, 1999).
Diversity and distribution of ECM root tips at
different scales
Although belowground root-tip analyses have been instrumental in furthering our understanding of ECM fungal
communities, it is without doubt that such studies are
confounded both by sampling intensity and by the scale at
which the study was conducted (Horton & Bruns, 2001;
Taylor, 2002). By constructing species area curves for data
published in four previous studies, Horton & Bruns (2001)
demonstrated that, in all but one, insufficient samples were
analysed to have covered the diversity of ECM taxa present.
This demonstrates that a potentially inaccurate or partial
FEMS Microbiol Rev ]] (2007) 1–19
5
Ectomycorrhizal fungal mycelia
picture of diversity and community composition can be
generated if the sampling intensity is insufficient. While this
alone has consequences for data interpretation, sampling
intensity cannot be considered independently from scale
(Lilleskov et al., 2004). This is particularly important
because in many field studies the desirable scale for the
analysis is the stand or forest level, but, in reality, pattern in
most ECM communities occurs at much finer scales. For
example, analysis of ECM root-tip community data from
eight studies suggested that it is necessary to take cores at
least 3 m apart in order to achieve the greatest sampling
efficiency for stand-level community analysis (Lilleskov
et al., 2004). However, ECM root-tip abundance and similarity of community composition have been shown to be
highly variable at much finer scales (5–20 cm) (Tedersoo
et al., 2003; Izzo et al., 2005), with a complete change in
ECM species composition being observed at a scale of 50 cm
in a mixed forest (Tedersoo et al., 2003).
There is an obvious trade-off between scale and sampling
intensity in root tip-based investigations of ECM communities, and alternative sampling approaches, including the
selection of random root tips from soil cores (Horton &
Bruns, 2001; Tedersoo et al., 2003), down to as few as three
ECM root-tips per sample (Koide et al., 2005b), have been
suggested as a possible way of tackling this problem. In most
ECM communities, a small number of ECM species are
highly abundant and dominant (Taylor, 2002). In these
situations it is difficult to see how this would not confound
the ecological interpretation of the data when so few root
tips are analysed.
Vertical stratification in ECM root-tip
communities
A major advance in our understanding of the belowground
ecology of ECM fungi has been the observation that ECM
root-tip communities are vertically stratified in the soil
profile, with different fungi typically occupying different
horizons (Goodman & Trofymow, 1998; Rosling et al., 2003;
Tedersoo et al., 2003; Izzo et al., 2005; Baier et al., 2006;
Genney et al., 2006). While this might signal a degree of
niche differentiation between ECM taxa, caution is required
in interpretation because the vertical distribution of ECM
roots does not necessarily reflect the distribution of mycelia
of the respective taxa (Genney et al., 2006; and see below).
Despite the extensive and often painstaking effort that has
been devoted to investigating communities of ECM roots, our
understanding of the belowground ecology of ECM fungi in
forest ecosystems remains limited. The ECM root-based
studies have provided unprecedented insights into the diversity of fungal taxa that form ECM associations, along with
aspects of their dynamics under a range of conditions, yet they
have failed to consider the components of ECM fungi that are
FEMS Microbiol Rev ]] (2007) 1–19
functionally most important in forest soil nutrient and carbon
cycling processes
the soil-borne mycelial systems.
Similar to aboveground and belowground comparisons
of ECM communities, numerous studies have reported a
lack of congruence between ECM species colonizing root
tips and those detected using molecular methods to be
present as mycelia in the same soil volume (Koide et al.,
2005a; Kjller, 2006). Therefore, the true diversity of ECM
species may remain uncovered if those species that are only
present as mycelia in a given soil volume, and which are
likely to be of some functional importance to their host
partners, are not considered. In addition, even where intense
sampling protocols have been adopted, the spatial distribution patterns of root-tip communities may not necessarily
reflect the patterns of the mycelial distribution in soil of the
constituent species (Genney et al., 2006).
Because ECM fungi are ‘nonresource-unit-restricted’
fungi (sensu Boddy, 1999), their mycelia forage through soil
to greater or lesser extents for nutrients, water and new
short-lateral roots to colonize (Read, 1992). In doing so,
they form indeterminate, structurally and physiologically
heterogeneous interconnected networks of which the colonized roots of the plant host form a part (Rayner, 1991;
Cairney & Burke, 1996). There is a growing awareness not
only that investigation of ECM mycelia in forest soils is
imperative to a functional understanding of ECM communities, but also that communities of ECM fungi when
considered from the perspective of their mycelial systems in
soil may be quite different from those identified on the basis
of colonized root tips (e.g. Horton & Bruns, 2001; Cairney,
2005; Anderson, 2006; Genney et al., 2006; Wallander, 2006).
Estimation of mycelial distribution in
ectomycorrhizal populations using root
tips
In addition to community analysis, ECM roots have been
used to estimate the distribution of ECM mycelial genotypes
belowground. This work has confirmed the observations
based on fruiting-body distribution (see above) that genotypes of some taxa are spatially restricted belowground and
that the distribution of others can be widespread (Guidot
et al., 2001; Hirose et al., 2004; Kretzer et al., 2004; Lian
et al., 2006). It is also clear that genotypes of some ECM
fungal taxa can infect multiple hosts in the field simultaneously (Lian et al., 2006), providing circumstantial support
for the notion of a common mycelial network in forest soil
(Peter, 2006). Importantly, it has also become evident that
while estimates of genotype distribution based on fruitingbody distribution sometimes correlate roughly with estimates based on root tips (Guidot et al., 2001; Zhou et al.,
2001b; Lian et al., 2006), in other instances root tip-based
estimates indicate more widespread genotype distribution
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
6
(Hirose et al., 2004). Furthermore, there is strong evidence
that belowground genotypes of at least some taxa, can differ
in temporal fruiting patterns (Guidot et al., 2001; Zhou
et al., 2001b; Hirose et al., 2004), meaning that, unless
repeated sampling is conducted, fruiting-body analysis may
only unveil part of the population that is present. Although,
with an appropriate sampling strategy, root-tip studies may
provide some information on the vertical distribution of
mycelial genotypes in ECM roots (Zhou et al., 2001b), they
do not necessarily provide an accurate reflection of the
vertical distribution of the equivalent soil-borne mycelia
(Genney et al., 2006).
Direct observation of ectomycorrhizal
mycelia in the field
The indeterminate filamentous nature of fungal hyphae,
coupled with the complex nature of soil fungal communities
(Fig. 1) and difficulties in identifying fungal mycelia in the
absence of fruiting bodies, makes direct observation and
identification of fungal mycelia in soil virtually impossible,
and ECM fungi are no exception in this regard (Bridge &
Spooner, 2001; Cairney, 2005). Some higher fungi produce
macroscopic linear mycelial aggregates (= rhizomorphs sensu
Cairney et al., 1991) during growth through soil. In the case
of certain saprotrophic fungi, robust rhizomorphs are
produced within the soil litter interface, and this has
facilitated excavation, direct measurement and mapping of
more-or-less intact mycelial systems (see Thompson, 1984).
Although many ECM fungi form rhizomorphs, these are
typically diminutive, and thus direct observations of ECM
mycelia in soil have been largely confined to those produced
within a few centimetres of ECM root tips or fruiting bodies.
From investigations of this nature, it is evident that different
ECM fungi produce different amounts of soil-borne mycelium, and that the propensity for mycelia to differentiate to
form rhizomorphs also varies considerably (reviewed by
Agerer, 2001). Mycelia that remain nonrhizomorphic are
thought to reflect a limited ability to explore surrounding
soil, while mycelia that comprise highly differentiated rhizomorphs are regarded as more adapted to long-distance
exploration (Agerer, 2001). Between the two extremes are a
range of more-or-less differentiated mycelial types that
facilitate medium-distance exploration in soil (Agerer,
2001). According to Agerer (2001), the most highly differentiated rhizomorphs can explore ‘up to several decimetres’
from the root surface in soil. Difficulties in excavating even
the most robust ECM rhizomorph systems in soil make it
difficult to determine the extent to which this underestimates the exploration potential of ECM mycelia in soil, but
nonetheless this classification scheme represents a useful
way to categorize ECM fungi based on the potential
ecological relevance of their mycelial systems.
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
I.C. Anderson et al.
Some ECM fungi produce dense mycelial mats or shiro in
forest soil. Mat-forming fungi such as Gaultheria and
Hysterangium spp. produce persistent dense mycelia that
occupy the upper few centimetres in soil and occupy areas
that are typically o 1 m2 (Cromack et al., 1979; Griffiths
et al., 1991). While discrete mats produced by these fungi
can be identified and measured in soil, it is not clear whether
they comprise single or multiple mycelia of the fungi in
question. The shiro produced by Tricholoma matsutake
persist for many years and develop by outward growth in a
‘fairy ring’ fashion from a central point for up to several
metres (Ogawa, 1977). Recent analysis of vegetative mycelia
from fruiting bodies indicate that a single shiro of
T. matsutake can comprise multiple mycelia (Murata et al.,
2005); however, the distribution of these mycelia within the
shiro remains unclear.
Observations of mycelia in microcosms
Our understanding of the physiological activities of ECM
mycelia in soil has been built largely on microcosm studies.
The most widely used microcosm system comprises a thin
layer of milled peat or soil between two Perspex sheets
(Duddridge et al., 1980), or a similar construction (Fig. 1).
Such microcosms have been used to elegantly demonstrate
the roles of ECM mycelia in the absorption and translocation of water, phosphorus and nitrogen from soil to the
plant host (Duddridge et al., 1980; Finlay & Read, 1986a, b;
Finlay et al., 1988, 1989; Ek et al., 1994; Andersson et al.,
1996; Timonen et al., 1996; Ek, 1997), along with establishing the mycelia as important conduits for carbon movement
from tree hosts into soil (Finlay & Read, 1986a, b; Ek, 1997;
Leake et al., 2001; Mahmood et al., 2001; Wu et al., 2002;
Heinonsalo et al., 2004; Rosling et al., 2004). The microcosms have also been pivotal in identifying potential patterns and processes involved in the growth, development
and differentiation of ECM mycelial systems (Read, 1992;
Donnelly et al., 2004), along with the likely influence of
edaphic changes such as pH (Erland et al., 1990; Ek et al.,
1994; Mahmood et al., 2001). An important observation
here has been the foraging behaviour of ECM mycelia in soil
and, in particular, their abilities to densely colonize discrete
patches of various organic substrates or necromass from
which they can effect nutrient mobilization (Finlay & Read,
1986a, b; Carleton & Read, 1991; Bending & Read, 1995a, b;
Pérez-Moreno & Read, 2000, 2001a, b; Leake et al., 2001;
Lilleskov & Bruns, 2003; Donnelly et al., 2004; Wallander &
Pallon, 2005). Microcosms have also been used to investigate
factors such as ECM mycelial longevity and temporal
changes in elemental composition (Downes et al., 1992;
Wallander & Pallon, 2005), interactions with soil minerals
(Wallander et al., 2002), responses of mycelia to elevated
atmospheric CO2 concentrations (e.g. Rouhier & Read,
FEMS Microbiol Rev ]] (2007) 1–19
7
Ectomycorrhizal fungal mycelia
1998, 1999; Fransson et al., 2005), spatial heterogeneity in
enzyme and gene expression in mycelial systems (Timonen
& Sen, 1998; Wright et al., 2005), and to quantify ECM
myelial area, density, biomass and elemental content in soil
(Ek, 1997; Schubert et al., 2003; Agerer & Raidl, 2004;
Donnelly et al., 2004; Hagerberg et al., 2005).
Although microcosm-based studies have facilitated significant advances in our appreciation of the activities of
ECM mycelia in soil, there is a danger that they may have
painted a somewhat unrealistic picture of the extent of ECM
mycelial system development in the field. Images of ECM
plants in microcosms generally portray a seedling with a
small root system and an ECM mycelial system that radiates
throughout much of the microcosm soil, exploring an area
of soil that is several times that occupied by the root system
(Fig. 1). The ‘extensive soil-colonizing vegetative mycelium’
produced in microcosms is viewed as representing an ideal
model of ECM systems in forest soil (Sen, 2000); however,
such extrapolation needs to be undertaken with caution for
several reasons. First, the extent of mycelial development in
more-or-less homogenous milled or sieved microcosm substrates is unlikely to reflect that through the heterogeneous
matrix of natural forest soil. Indeed, as highlighted by Smith
& Read (1997), the intensive hyphal colonization observed
in patches of introduced organic matter (e.g. Finlay & Read,
1986a, b; Bending & Read, 1995b; Pérez-Moreno & Read,
2000; Leake et al., 2001) may be more representative of
mycelial growth in forest soil than the extensive mycelial
exploration that occurs through the homogeneous microcosm substrates. The largely two-dimensional nature of the
microcosms means that exploration of the substrate is
effectively on a single plane, and thus the distance that
mycelium grows from the root system is likely to be greater
than would be the case for the equivalent mycelial biomass
in a three-dimensional soil mass. Even where three-dimensional microcosms have been used (e.g. Coutts & Nicholl,
1990), however, observations of mycelia rely on growth at
the interface between the soil and the transparent microcosm window, and, as such, are unlikely to reflect growth of
the mycelia within the soil column. Add to this the lack of
vertical stratification of soil (see below) in the microcosms
(although the rudimentary soil profile reconstruction of
Heinonsalo et al. (2004) partially addressed this), along with
the small size of seedlings, for which the carbon balance and
patterns of carbon allocation are likely to be dissimilar to
that of mature trees in the field (Ericsson et al., 1996), and
the relative growth of mycelia in microcosms may not be
much of a guide to mycelia in the field.
Despite these limitations, rates of growth of ECM mycelia
in microcosms (Read, 1992) have been used to estimate rates
of mycelial expansion in the field (e.g. Bonello et al., 1998;
Sawyer et al., 1999). With the above in mind, and the fact
that the nature of the substrate and edaphic conditions can
FEMS Microbiol Rev ]] (2007) 1–19
strongly affect ECM mycelial growth (Erland et al., 1990;
Smith & Read, 1997), such estimation must be viewed with a
degree of caution. Attention must also be drawn to the fact
that, in most cases, microcosms reflect mycelial growth of a
single ECM fungus in the absence of competing ECM or
other macro fungi. Where competing ECM fungi have been
coinoculated in microcosms, it is clear that mycelial development can be altered significantly (Wu et al., 1999). While
the outcomes of interactions appear to vary, largely according to relative carbon availability to the individual fungi
(Lindahl, 2000; Lindahl et al., 2001), it is further evident that
the presence of saprotrophic basidiomycete mycelia can
influence the growth and development of ECM mycelia in
microcosms (Lindahl et al., 1999, 2001; Leake et al., 2001,
2002). Because mycelia of different taxa may preferentially
grow at different depths in the soil (Lindahl et al., 1999), the
largely two-dimensional nature of the microcosms will
reduce the potential for different mycelia to avoid competition by vertical stratification of growth (Cairney, 2005). Care
must therefore be taken when attempting to extrapolate
outcomes of competitive interactions between mycelia in
microcosms to the field.
Estimation of mycelial biomass in the field
Given the apparent importance of ECM mycelia in forest
carbon and nutrient cycles, an understanding of their
belowground biomass is pivotal to fully appreciating their
contributions to biogeochemical processes. While estimates
of mycelial biomass in simple nonsoil substrates have been
obtained in the laboratory (e.g. Colpaert et al., 1992;
Rousseau et al., 1994), determining ECM mycelial biomass
in field soil has, until recently, remained a vexed issue.
Högberg & Högberg (2002) adopted an indirect method
that involved tree girdling, soil fumigation and measurements of dissolved organic carbon to estimate that ECM
mycelium accounts for 4 32% of the total microbial
biomass in a boreal forest, while estimates have also been
derived by long-term incubation of soil in the laboratory.
Incubation in the absence of a host plant means that ECM
mycelia should degrade during the incubation period, and
thus the difference between fungal biomass, estimated by
ergosterol or phospholipid fatty acid (PLFA) analysis, before
and after incubation, is used as an estimate of ECM fungal
biomass (Wallander et al., 2004). Such studies indicate that
ECM mycelial biomass decreases with soil depth (Bååth
et al., 2004; Wallander et al., 2004).
Hyphal ingrowth bags offer a more direct method for
analysis of ECM mycelial growth and biomass in soil. These
are nylon mesh (50 mm) bags containing acid-washed sand
that are buried in soil and later retrieved for analysis of total
fungal content by ergosterol or PLFA analysis (Wallander
et al., 2001). The absence of organic matter in the sand
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
8
substrate is thought to select for mycelia of ECM fungi over
saprotrophs, because ECM fungi have a carbon source from
their tree hosts (Wallander et al., 2001). Comparison of
mycelial biomass in hyphal ingrowth bags from trenched
plots (designed to sever ECM roots from their tree hosts,
and so exclude ECM mycelia from the plots) with that from
untrenched plots suggests that some 85–90% of the mycelium that colonizes the sand-filled bags in Swedish forest
soils is ECM (Wallander et al., 2001; Hagerberg & Wallander,
2002; Nilsson & Wallander, 2003). This is supported by d13C
values derived from the bags (Wallander et al., 2001), along
with molecular analysis of the fungal taxa present as mycelia
in hyphal ingrowth bags from a range of forest habitats
(Wallander et al., 2003; Bastias et al., 2006; Kjller, 2006 and
see below). The method relies upon the assumptions that the
use of acid-washed sand provides a reasonable approximation of mycelial growth in native soil, and that there is no
turnover of mycelium during the period in which bags are
buried in soil (Wallander et al., 2001; Hendricks et al., 2006).
Recent data suggest that ECM mycelial colonization of bags
containing acid-washed sand can be considerably reduced
compared with that of native soil, indicating that the former
may underestimate ECM biomass in the surrounding soil, a
fact that may be exacerbated if mycelial turnover occurs
during the burial period (Hendricks et al., 2006). The use of
soil as a substrate in ingrowth bags, however, requires that
there is relatively low fungal biomass in the soil (Wallander,
2006), and further work on the influence of substrate is
clearly required. It has also been suggested that ingrowth
bags may select for certain ECM fungal taxa that are able to
colonize the substrate rapidly at the expense of slowergrowing taxa (Wallander et al., 2003).
Despite the various questions regarding the efficacy of
ingrowth bags for investigation of ECM mycelia, this
method remains the most direct for estimation of ECM
mycelial biomass in the field. Work of this nature indicates
that ECM fungal biomass in coniferous forests can be of the
order of 100–600 kg ha 1 and that it comprises a substantial
part of the soil fungal biomass (Wallander et al., 2001, 2004;
Hagerberg et al., 2003; Hendricks et al., 2006). Growth of
ECM mycelium appears to be seasonal, with greatest activity
in autumn and little or no growth during winter (Wallander
et al., 2001; Hagerberg & Wallander, 2002). ECM mycelial
biomass has also been shown to vary with soil depth and
host tree species, being generally greater in the upper part of
the soil profile than in the underlying soil and correlated
with the distribution of tree roots in the profile (Wallander
et al., 2004; Göransson et al., 2006). It is also evident that
ECM mycelial biomass in forest soil can be negatively
influenced by soil nutrient, particularly nitrogen, status
(Nilsson & Wallander, 2003; Nilsson, 2004; Nilsson et al.,
2005; Hendricks et al., 2006). In contrast, fertilization of
arctic tundra has recently been shown to significantly
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
I.C. Anderson et al.
increase ECM mycelial biomass (Clemmensen et al., 2006).
The extent to which these observations reflect altered
mycelium production per se, or a shift in the ECM fungal
community towards taxa that produce more/less soil-borne
mycelium, however, remains unclear. It is also evident that
ECM mycelial production can be influenced by temperature
(Clemmensen et al., 2006), and that mycelia can grow in
response to certain soil minerals (Hagerberg & Wallander,
2002; Hagerberg et al., 2003) and can contribute to the
mobilization of minerals such as apatite (Wallander et al.,
2002, 2003).
Analysis of ECM communities by direct
DNA extraction
In recent years methods have been developed to allow
analysis of microbial communities by direct nucleic acid
extraction from soil. These techniques include cloning,
denaturing gradient gel electrophoresis (DGGE), temperature gradient gel electrophoresis (TGGE) and terminal
restriction fragment length polymorphism (T-RFLP). The
technical considerations and limitations associated with
these approaches in the context of both general soil (Anderson & Cairney, 2004; Bidartondo & Gardes, 2005) and ECM
(Anderson, 2006) fungal communities have been reviewed
recently and thus will not be considered here.
Direct DNA extraction from soil
Recent investigations based on direct DNA extraction from
soil have yielded information on the diversity and distribution of ECM mycelia in soil (Table 1). Although they did not
target ECM fungi specifically, several studies identified
mycelia of ECM fungi as being present in the general fungal
assemblages of forest soils (Chen & Cairney, 2002; Anderson
et al., 2003; Landeweert et al., 2003a). Given that the
majority of ECM fungi are basidiomycetes (Smith & Read,
1997), basidiomycete-specific PCR primers have been used
to increase the detection of ECM mycelia in soil DNA
extracts. Using this method, Landeweert et al. (2003a)
reported vertical stratification of certain ECM fungi in the
soil profile, while Smit et al. (2003) demonstrated that
removal of litter and humus layers from a pine plantation
increased the diversity of ECM mycelia. However, the use
of basidiomycete-specific primers for the analysis of
ECM mycelial communities has been cautioned (Anderson,
2006), because the diversity (e.g. Vrålstad et al., 2002;
Tedersoo et al., 2006) and abundance (e.g. Koide et al.,
2005a) of ascomycetes in ECM root-tip communities is
often high.
An alternative means of targeting ECM fungi in DNA
extracted directly from soil is T-RFLP analysis and interrogation of a database containing reference terminal fragments of known ECM fungi from the site. This has
FEMS Microbiol Rev ]] (2007) 1–19
9
Ectomycorrhizal fungal mycelia
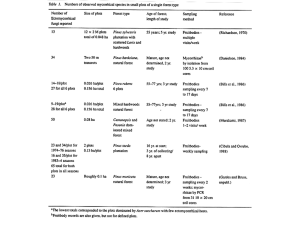
Table 1. Investigations of soil ECM mycelial communities using direct DNA extraction and molecular analysis methods
Forest type
DNA extracted from
Analysis method
Target fungal group
Reference
Larix kaempferi forest
Sclerophyll forest
Pinus resinosa plantation
Pinus pinaster forest
Pinus sylvestris forest
Coniferous forest
Pinus sylvestris plantation
Pinus taeda plantation
Pinus pinaster forest
Pinus resinosa plantation
Pinus resinosa plantation
Pinus sylvestris forest
Wet sclerophyll forest
Pinus sylvestris plantation
Fagus sylvatica forest
Picea abies plantation
Pinus sylvestris forest
Soil and ECM roots
Soil
Soil
Soil
Soil
Soil
Soil
Soil
Soil
Soil and ECM roots
Soil and ECM roots
Soil and ECM roots
Hyphal ingrowth bags
Soil and ECM roots
Hyphal ingrowth bags
Hyphal ingrowth bags
Soil
Simple sequence repeat analysis
Cloning/sequencing
T-RFLP
Competitive PCR
DGGE/sequencing
Cloning/sequencing
DGGE and cloning/sequencing
T-RFLP
Competitive PCR
T-RFLP
T-RFLP
DGGE/sequencing
DGGE and cloning/sequencing
T-RFLP
Cloning/sequencing
DGGE/sequencing
T-RFLP and cloning/sequencing
Suillus grevillei
Soil fungi
ECM
Hebeloma cylindrosporum
Soil fungi
Basidiomycetes
Basidiomycetes
Basidiomycetes
Hebeloma cylindrosporum
ECM
ECM
Basidiomycetes
ECM
ECM
ECM
ECM
ECM and saprotrophic fungi
Zhou et al. (2001b)
Chen & Cairney (2002)
Dickie et al. (2002)
Guidot et al. (2002)
Anderson et al. (2003)
Landeweert et al. (2003a)
Smit et al. (2003)
Edwards et al. (2004)
Guidot et al. (2004)
Koide et al. (2005a)
Koide et al. (2005b)
Landeweert et al. (2005)
Bastias et al. (2006)
Genney et al. (2006)
Kjller (2006)
Korkama et al. (2007)
Lindahl et al. (2007)
facilitated confirmation of the vertical stratification of ECM
mycelial communities in the soil profile (Dickie et al., 2002;
Genney et al., 2006). More importantly, Koide et al. (2005b)
demonstrated that positive or negative interspecific interactions can influence the distribution of ECM mycelia in soil
and that such interactions can vary according to soil
conditions.
T-RFLP analysis of DNA extracted from multiple adjacent
soil cores allowed Edwards et al. (2004) to estimate the
minimum width of mycelial patches of several ECM taxa in a
horizontal plane, the widest patch-size recorded being
160 cm for Thelephora terrestris. Moreover, Genney et al.
(2006) coupled T-RFLP analysis with a soil-slicing approach
to investigate the distribution of ECM mycelia in a volume
of soil (800 cm3) at a fine scale. Mycelial patches of individual ECM taxa were variously shown to occupy different
volumes up to a maximum of 312 cm3 for Cadophora
finlandica. A combination of T-RFLP analysis and cloning
has also been used to demonstrate vertical separation of
ECM and saprotrophic mycelia in a forest-soil profile
(Lindahl et al., 2007). While each of these studies demonstrated the potential distribution of mycelia of various ECM
species in forest soil, none considered the distribution
of individual mycelia ( = genet) of the species. Zhou et al.
(2001a, b), however, have demonstrated that simple sequence repeat (SSR) markers can be applied to investigate
the distribution of individual mycelia of Suillus grevillei
following direct DNA extraction from soil. With the increasing development of SSR markers for ECM fungi (e.g.
Bergemann & Miller, 2002; Kretzer et al., 2004; Hitchcock
et al., 2006), information regarding the distribution of
individual mycelia within the soil volume should increase
markedly in the near future.
FEMS Microbiol Rev ]] (2007) 1–19
Even where individual mycelia of ECM fungal taxa can be
identified in soil, observations are restricted to determining
only the presence or absence of the mycelium in a sampled
soil volume, and the methods provide no information on
mycelial density. Competitive PCR, however, offers a means
of achieving this goal. Indeed, Guidot et al. (2002, 2004)
have used this approach successfully to show that soil-borne
mycelia of Hebeloma cylindrosporum are ephemeral, extend
for o 50 cm, and decrease in density with increasing
distance from the base of basidiomes.
A fundamental assumption in the interpretation of data
generated by direct DNA extraction from soil is that the taxa
identified are present as mycelia rather than as spores or
other resting propagules. Although approaches such as the
collection of soil samples after basidiome production has
ceased (Dickie et al., 2002) have been used to minimize the
likely detection of spore DNA, the fact that DNA from a
small number of H. cylindrosporum spores can be detected
in soil DNA extracts (Guidot et al., 2004) demonstrates
the potential for spore DNA to be amplified. Given that
dormant propagules are likely to contain little RNA relative
to active mycelia, RNA-based approaches (e.g. Anderson &
Parkin, 2006) may provide a more robust means to detect
active ECM mycelia in soil in the future.
The importance of analysing ECM fungal communities
by direct DNA extraction from soil is emphasized by the lack
of congruence between community structures inferred from
root-tip and soil-based analyses (Koide et al., 2005a; Landeweert et al., 2005). Furthermore, it is evident that spatial
segregation can exist between root tips and mycelia of
individual ECM taxa in the soil profile, with abundant
mycelium of some species occurring in the absence of
infected roots tips (Genney et al., 2006). Indeed, the same
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
10
appears to be true for individual mycelia of S. grevillei (Zhou
et al., 2001b).
DNA extraction from hyphal ingrowth bags
Hyphal ingrowth bags, in conjunction with ergosterol and/
or PLFA analysis, has been the main method used to
measure mycelial growth and biomass in forest soil (see
above). More recently, however, the approach has been
combined with direct DNA extraction from the ingrowthbag substrate and molecular methods to identify the mycelia
of ECM fungal taxa colonizing the bags. Regardless of the
molecular method used, these studies indicated that
83–91% of taxa colonizing hyphal ingrowth bags in a wet
sclerophyll forest (Bastias et al., 2006), a Fagus sylvatica
forest (Kjller, 2006) or a Picea abies plantation (Korkama
et al., 2007) were ECM fungi, which is broadly similar to
hyphal ingrowth bag-based estimates of ECM fungal biomass in Swedish forest soils using d13C measurements and
trenching (Wallander et al., 2001; Hagerberg & Wallander,
2002; Nilsson & Wallander, 2003).
The hyphal ingrowth-bag approach has been successfully
used in conjunction with DGGE to investigate the effects of
plant host and forest management practices on ECM
mycelial communities. Thus, Korkama et al. (2007) demonstrated that fast-growing Picea abies clones support different
ECM fungal mycelial communities from slower-growing
clones, with Atheliaceae taxa more abundant in the former.
Bastias et al. (2006) used this method to establish that longterm repeated prescribed burning of wet sclerophyll forest
can significantly alter the composition of mycelial communities of ECM fungi in the upper 10 cm of the soil profile.
Cloning and sequencing of DNA extracted from the ingrowth bags further suggested that frequent burning had a
strong negative effect on Cortinariaceae and a positive effect
on Thelephoraceae taxa (Bastias et al., 2006). Comparison of
ECM fungi colonizing hyphal ingrowth bags with those
species colonizing root tips adjacent to the ingrowth bags
in a Fagus sylvatica forest showed that Boletoid species
occurred more frequently as mycelia than in root tips, in
contrast to Russuliod and Cortinarius spp., for which the
opposite was true (Kjller, 2006). It should be noted that, in
each of these investigations, only sand was used as substrate
in the ingrowth bags. Because the nature of the substrate can
influence mycelial colonization of hyphal ingrowth bags
(Hendricks et al., 2006), it may also bias community
composition. To date there is no published comparison
between mycelia of ECM fungi colonizing sand-filled hyphal
ingrowth bags with mycelia in the surrounding soil.
A combination of PLFA analysis and molecular approaches allows determination of both mycelial biomass
and ECM species identity in hyphal ingrowth bags (Korkama et al., 2007), but it is difficult to correlate these two
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
I.C. Anderson et al.
independent measurements directly. Correlation of both
relative abundance (community evenness) and species identity (species richness) is important for determining the
mycelial biomass of individual ECM species and the response of individual ECM taxa in manipulative field experiments. This approach is common in root tip-based
community studies of ECM fungi, although the limitations
of root tip-based measurements of relative abundance have
been previously discussed (Taylor, 2002). While determination of the relative abundance of mycelia of individual ECM
species is yet to be achieved, this should be possible in the
future by combining quantitative molecular methods such
as competitive PCR (e.g. Guidot et al., 2002, 2004) or realtime quantitative PCR (Landeweert et al., 2003b; Schubert
et al., 2003; Raidl et al., 2005) with the hyphal ingrowth-bag
approach. Given the known variation in the growth form of
mycelial systems of different ECM species (Agerer, 2001),
this will be an important future development in the use of
hyphal ingrowth bags.
ECM mycelia and climate change
The importance of understanding the diversity, extent and
dynamics of ECM mycelial systems in forest soils is emphasized by current interest in belowground responses to
elevated atmospheric CO2 in the context of global climate
change (Pendall et al., 2004) and the need to understand
both plant and ECM fungal responses (Alberton et al.,
2005). ECM fungal mycelia can comprise 80% of the total
fungal biomass and 30% of the microbial biomass in some
forest soils (Wallander et al., 2001, 2003; Högberg &
Högberg, 2002), with carbon allocation to ECM fungi
estimated to be as much as 22% of net primary production
(Hobbie, 2006). ECM fungi are thus an important component of forest carbon cycles, and the effects of elevated
atmospheric CO2 on these fungi have deservedly received
recent attention. Much of this work has focused on interactions between CO2 concentration and root colonization by
ECM fungi. With one exception (Rouhier & Read, 1999),
such investigations have reported increased percentage root
colonization by ECM fungi under elevated atmospheric CO2
conditions (Norby et al., 1987; Ineichen et al., 1995;
Berntson et al., 1997; Godbold et al., 1997; Tingey et al.,
1997; Rouhier & Read, 1998; Walker et al., 1998; Kasurinen
et al., 2005).
Elevated atmospheric CO2 has also been shown to alter
ECM fungal root-tip community structure in growthchamber experiments (Godbold & Berntson, 1997; Godbold
et al., 1997; Rey & Jarvis, 1997; Rygiewicz et al., 2000) and
in the field (Fransson et al., 2001; Kasurinen et al., 2005). In
one such experiment, a change in ECM root-tip community
composition in favour of morphotypes that appeared to
produce emanating hyphae and/or rhizomorphs was noted
FEMS Microbiol Rev ]] (2007) 1–19
11
Ectomycorrhizal fungal mycelia
Table 2. Responses of ECM fungal mycelia to elevated atmospheric CO2 concentrations
Fungus
Host tree
Experimental system
Mycelial response
Reference
Hebeloma crustuliniforme
Laccaria bicolor
Paxillus involutus
Paxillus involutus
Pisolithus tinctorius
Suillus bovinus
Suillus bovinus
ECM mycelial community
Pinus sylvestris
Pinus sylvestris
Pinus sylvestris
Betula pendula
Pinus sylvestris
Pinus sylvestris
Pinus sylvestris
Betula pendula
Pot experiment
Petri-dish microcosm
Perspex microcosm
Perspex microcosm
Petri-dish microcosm
Perspex microcosm
Petri-dish microcosm
Field experiment
(hyphal ingrowth bags)
Increased mycelial biomass (300%)
No effect on hyphal length
Increased mycelial spread (4 400%)
Increased mycelial spread (30%)
Increased mycelial biomass (200%)
Increased mycelial spread (200%)
No effect on hyphal length
No effect on mycelial biomass
Fransson et al. (2005)
Gorissen & Kuyper (2000)
Rouhier & Read (1998)
Rouhier & Read (1999)
Ineichen et al. (1995)
Rouhier & Read (1998)
Gorissen & Kuyper (2000)
Kasurinen et al. (2005)
under elevated atmospheric CO2 conditions (Godbold &
Bernston, 1997; Godbold et al., 1997). Because no attempt
was made to quantify soil-borne mycelia, however, this
provides no direct information on the mycelial response of
ECM fungi under these conditions. Several laboratory-based
microcosm experiments have been undertaken with a view
to determining the responses of ECM mycelia to elevated
atmospheric CO2 (Table 2). While Gorissen & Kuyper
(2000) observed no significant increase in mycelial production, other studies have reported increases in mycelial
biomass or spread of up to 400% (Ineichen et al., 1995;
Rouhier & Read, 1998, 1999; Fransson et al., 2005). It should
be emphasized that these investigations were conducted in
simplified microcosm systems (see above) using seedlings
inoculated with a single ECM fungus. As such, the observed
responses may not reflect the responses of ECM mycelia to
elevated atmospheric CO2 in the field, highlighting the need
for field-based investigations. Although elevated atmospheric CO2 was found to alter the structure of a basidiomycete mycelial community (that included ECM fungi) in a
scrub oak forest (Klamer et al., 2002), to date only one such
study has focused on ECM fungi. By analysing PLFAs from
hyphal ingrowth bags buried under Betula pendula trees in
open-top chambers, Kasurinen et al. (2005) established that
elevated atmospheric CO2 had no significant effect on
mycelial production by ECM fungi.
The inability to generalize based on this single field
experiment notwithstanding, these data relate only to the
response of the ECM mycelial community and therefore provide no information on responses of individual
ECM species to elevated atmospheric CO2 in the field.
Further studies on how mycelial systems of ECM fungi
respond to elevated atmospheric CO2 concentrations will
be crucial to modelling and understanding carbon dynamics
in complex forest ecosystems under future climate-change
scenarios.
Conclusions
Soil-borne mycelia of ECM fungi are a dominant and
important component of soil microbial communities in
FEMS Microbiol Rev ]] (2007) 1–19
forest ecosystems, where they play crucial roles in nutrient
and carbon cycling processes. Our appreciation of the roles
of ECM fungi in these processes is hampered by a fundamental lack of understanding of their mycelial systems in
soil. Much of the currently available information on the
diversity, distribution and dynamics of ECM fungi is derived
from investigations of fruiting bodies and ECM root tips,
and provides only limited insights into soil-borne ECM
mycelial communities. Information derived from microcosm studies, while unarguably enhancing our understanding of ECM mycelial functioning, cannot necessarily be
extrapolated directly to mycelia in the field, emphasizing
the necessity for direct analysis of soil-borne mycelia of
ECM fungi in situ. Molecular approaches, combined with
new experimental approaches, such as hyphal ingrowth
bags, facilitate analysis of ECM fungal communities from
the perspective of mycelia in soil. Furthermore, they have
the potential to increase our understanding of the distribution of individual mycelia and so enhance our appreciation
of the physical and physiological continuity of mycelial
systems in soil. Such information will give unprecedented
insights into the functioning of ECM mycelia at the ecosystem level and will assume particular importance when
considering the responses of ECM fungi to future perturbations, particularly in the context of land-use changes and
global climate change.
Acknowledgements
This work was supported by an Australian Research Council
Linkage International Awards grant (LX0455012) and a
Macaulay Development Trust collaboration grant. I.C.A.
receives funding from the Scottish Executive Environment
and Rural Affairs Department.
References
Agerer R (1987–2002) Colour Atlas of Ectomycorrhizae. EinhornVerlag, Munich.
Agerer R (2001) Exploration types of ectomycorrhizae. A
proposal to classify ectomycorrhizal mycelial systems
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
12
according to their patterns of differentiation and putative
ecological importance. Mycorrhiza 11: 107–114.
Agerer R & Raidl S (2004) Distance-related semi-quantitative
estimation of the extramatrical ectomycorrhizal mycelia of
Cortinarius obtusus and Tylospora asterophora. Mycol Progress
3: 57–64.
Alberton O, Kuyper TW & Gorissen A (2005) Taking
mycocentrism seriously: mycorrhizal fungal and plant
responses to elevated CO2. New Phytol 167: 859–868.
Anderson IC (2006) Molecular ecology of ectomycorrhizal fungal
communities: new frontiers. Molecular Approaches to Soil,
Rhizosphere and Plant Microorganism Analysis (Cooper JE &
Rao JR, eds), pp. 183–197. CAB International, Wallingford,
UK.
Anderson IC & Cairney JWG (2004) Diversity and ecology of soil
fungal communities: increased understanding through the
application of molecular techniques. Environ Microbiol 6:
769–779.
Anderson IC & Parkin PI (2007) Detection of active soil fungi by
RT-PCR amplification of precursor rRNA molecules.
J Microbiol Methods 68: 248–253.
Anderson IC, Chambers SM & Cairney JWG (1998) Use of
molecular methods to estimate the size and distribution of
mycelial individuals of the ectomycorrhizal basidiomycete
Pisolithus tinctorius. Mycol Res 102: 295–300.
Anderson IC, Chambers SM & Cairney JWG (2001) Distribution
and persistence of Australian Pisolithus spp. genets at native
sclerophyll forest field sites. Mycol Res 105: 971–976.
Anderson IC, Campbell CD & Prosser JI (2003) Diversity of fungi
in organic soils under a moorland – Scots pine (Pinus sylvestris
L.) gradient. Environ Microbiol 5: 1121–1132.
Andersson S, Jensén P & Söderström B (1996) Effects of
mycorrhizal colonization by Paxillus involutus on uptake of CA
and P by Picea abies and Betula pendula grown in unlimed and
limed peat. New Phytol 133: 695–704.
Avis PG, McLaughlin DJ, Dentinger BC & Reich PB (2003) Longterm increase in nitrogen supply alters above- and
belowground ectomycorrhizal communities and increases the
dominance of Russula spp. in a temperate oak savanna. New
Phytol 160: 239–253.
Baar J, Ozinga WA & Kuyper TW (1994) Spatial distribution of
Laccaria bicolor genets reflected by sporocarps after removal of
litter and humus layers in a Pinus sylvestris forest. Mycol Res 98:
726–728.
Bååth E, Nilsson LO, Göransson H & Wallander H (2004) Can the
extent of degradation of soil fungal mycelium during soil
incubation be used to estimate ectomycorrhizal biomass in
soil? Soil Biol Biochem 36: 2105–2109.
Bagley SJ & Orlovich DA (2004) Genet size and distribution of
Amanita muscaria in a suburban park, Dunedin, New Zealand.
New Zeal J Bot 42: 939–947.
Baier R, Ingenhaag J, Blaschke H, Göttlein A & Agerer R (2006)
Vertical distribution of an ectomycorrhizal community in
upper soil horizons of a young Norway spruce (Picea abies [L.]
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
I.C. Anderson et al.
Karst.) stand in the Bavarian Limestone Alps. Mycorrhiza 16:
197–206.
Bastias BA, Xu Z & Cairney JWG (2006) Influence of repeated
long-term repeated prescribed burning on mycelial
communities of ectomycorrhizal fungi. New Phytol 172:
149–158.
Bending GD & Read DJ (1995a) The structure and function of the
vegetative mycelium of ectomycorrhizal plants. V. Foraging
behaviour and translocation of nutrients from exploited litter.
New Phytol 130: 401–409.
Bending GD & Read DJ (1995b) The structure and function of
the vegetative mycelium of ectomycorrhizal plants. VI.
Activities of nutrient mobilizing enzymes in birch litter
colonized by Paxillus involutus (Fr.) Fr. New Phytol 130:
411–417.
Berch SM, Brockley RP, Battigelli JP, Hagerman S & Holl B (2006)
Impacts of repeated fertilization on components of the soil
biota under a young lodgepole pine stand in the interior of
British Columbia. Can J For Res 36: 1415–1426.
Bergemann SE & Miller SL (2002) Size, distribution, and
persistence of genets in local populations of the late-stage
ectomycorrhizal basidiomycete, Russula brevipes. New Phytol
156: 313–320.
Bergemann SE, Douhan GW, Garbaletto M & Miller SL (2006)
No evidence of population structure across three isolated
subpopulations of Russula brevipes in an oak/pine woodland.
New Phytol 170: 177–184.
Berntson GM, Wayne PM & Bazzaz FA (1997) Belowground
architectural and mycorrhizal responses to elevated CO2 in
Betula alleghaniensis populations. Funct Ecol 11: 684–695.
Bidartondo MI & Gardes M (2005) Fungal diversity in molecular
terms: profiling, identification, and quantification in the
environment. The Fungal Community. 3rd edn (Dighton J,
White JF & Oudemans P, eds), pp. 215–239. CRC Press, Boca
Raton, FL.
Boddy L (1999) Saprotrophic cord-forming fungi: meeting the
challenge of heterogeneous environments. Mycologia 91:
13–32.
Bonello P, Bruns TD & Gardes M (1998) Genetic structure of a
natural population of the ectomycorrhizal fungus Suillus
pungens. New Phytol 138: 533–542.
Bridge P & Spooner B (2001) Soil fungi: diversity and detection.
Plant Soil 232: 147–154.
Brown JH, Whitham TG, Morgan Ernest SK & Gehring CA
(2001) Complex species interactions and the dynamics of
ecological systems: long-term experiments. Science 293:
643–650.
Brundrett M, Bougher N, Dell B, Grove T & Malajczuk N (1996)
Working with Mycorrhizas in Forestry and Agriculture.
Australian Centre for International Agricultural Research,
Canberra.
Cairney JWG (2005) Basidiomycete mycelia in forest soils:
dimensions, dynamics and roles in nutrient distribution.
Mycol Res 109: 7–20.
FEMS Microbiol Rev ]] (2007) 1–19
13
Ectomycorrhizal fungal mycelia
Cairney JWG & Burke RM (1996) Physiological heterogeneity
within fungal mycelia: an important concept for a functional
understanding of the ectomycorrhizal symbiosis. New Phytol
134: 685–695.
Cairney JWG & Meharg AA (1999) Influences of anthropogenic
pollution on mycorrhizal fungal communities. Environ Pollut
106: 169–182.
Cairney JWG & Bastias BA (2007) Influences of fire on forest soil
fungal communities. Can J For Res 37: 207–215.
Cairney JWG, Jennings DH & Agerer R (1991) The nomenclature
of fungal multi-hyphal linear aggregates. Crypt Bot 2/3:
246–251.
Carfrae JA, Skene KR, Sheppard LJ, Ingleby K & Crossley A (2006)
Effects of nitrogen with and without acidified sulphur on an
ectomycorrhizal community in a Sitka spruce (Picea sitchensis
Bong. Carr) forest. Environ Pollut 141: 131–138.
Carleton TJ & Read DJ (1991) Ectomycorrhizas and nutrient
transfer in conifer–feather moss ecosystems. Can J Bot 69:
778–785.
Chambers SM, Hitchcock CJ & Cairney JWG (2005)
Ectomycorrhizal mycobionts of Pisonia grandis on coral cays
in the Capricorn-Bunker group, Great Barrier Reef, Australia.
Mycol Res 109: 1105–1111.
Chen DM & Cairney JWG (2002) Investigation of the influence of
prescribed burning on ITS profiles of ectomycorrhizal and
other soil fungi at three Australian sclerophyll sites. Mycol Res
106: 532–540.
Clemmensen KE, Michelsen A, Jonasson S & Shaver GR (2006)
Increased ectomycorrhizal fungal abundance after long-term
fertilization and warming of two arctic tundra ecosystems.
New Phytol 171: 391–404.
Colpaert JV, Van Assche JA & Luijtens K (1992) The growth of the
extramatrical mycelium of ectomycorrhizal fungi and the
growth response of Pinus sylvestris L. New Phytol 120: 127–135.
Conn C & Dighton J (2000) Litter quality influences
decomposition, ectomycorrhizal community structure and
mycorrhizal root surface acid phosphatase activity. Soil Biol
Biochem 32: 489–496.
Coutts MP & Nicholl BC (1990) Growth and survival of shoots,
roots and mycorrhizal mycelium in clonal Sitka spruce during
the first growing season after planting. Can J For Res 20:
861–868.
Cullings K, Raleigh C & Vogler DR (2005) Effects of severe dwarf
mistletoe infection on the ectomycorrhizal community of a
Pinus contorta stand in Yellowstone Park. Can J Bot 83:
1174–1180.
Cullings KW, New MH, Makhija S & Parker VT (2003) Effects of
litter addition on ectomycorrhizal associates of a lodgepole
pine (Pinus contorta) stand in Yellowstone National Park. Appl
Environ Microbiol 69: 3772–3776.
Cromack K, Sollins P, Graustein WC, Speidel K, Todd AW,
Spycher G, Li CY & Todd RL (1979) Calcium oxalate
accumulation and soil weathering in mats of the hypogeous
fungus Hysterangium crassum. Soil Biol Biochem 11: 463–468.
FEMS Microbiol Rev ]] (2007) 1–19
Dahlberg A (1997) Population ecology of Suillus variegatus in old
Swedish Scots pine forests. Mycol Res 101: 47–54.
Dahlberg A & Stenlid J (1990) Population structure and dynamics
in Suillus bovinus as indicated by spatial distribution of fungal
clones. New Phytol 115: 478–493.
Dahlberg A & Stenlid J (1994) Size, distribution and biomass of
genets in populations of Suillus bovinus (L.: Fr.) Roussel
revealed by somatic incompatibility. New Phytol 128: 225–234.
Dahlberg A & Stenlid J (1995) Spatiotemporal patterns in
ectomycorrhizal populations. Can J Bot 73(Suppl):
S1222–S1230.
De la Bastide PY, Kropp BR & Piché Y (1994) Spatial distribution
and temporal persistence of discrete genotypes of the
ectomycorrhizal fungus Laccaria bicolor (Maire) Orton. New
Phytol 127: 547–556.
Dickie IA & Reich PB (2005) Ectomycorrhizal fungal
communities at forest edges. J Ecol 93: 244–255.
Dickie IA, Xu B & Koide RT (2002) Vertical niche differentiation
of ectomycorrhizal hyphae in soil as shown by T-RFLP
analysis. New Phytol 156: 527–535.
Dighton J, Tuininga AR, Gray DM, Huskins RE & Belton T
(2004) Impacts of atmospheric deposition on New Jersey pine
barrens forest soils and communities of ectomycorrhizae.
Forest Ecol Manag 201: 133–144.
Donnelly DP, Boddy L & Leake JR (2004) Development,
persistence and regeneration of foraging ectomycorrhizal
mycelial systems in soil microcosms. Mycorrhiza 14: 37–45.
Downes GM, Alexander IJ & Cairney JWG (1992) A study of
ageing of spruce [Picea sitchensis (Bong.) Carr.]
ectomycorrhizas. I. Morphological and cellular changes in
mycorrhizas formed by Tylospora fibrillosa (Burt.) Donk and
Paxillus involutus (Batsch. ex Fr.) Fr. New Phytol 122: 141–152.
Duddridge JA, Malibari A & Read DJ (1980) Structure and
function of mycorrhizal rhizomorphs with special reference to
their role in water transport. Nature 287: 834–836.
Dunham SM, Kretzer A & Pfrender ME (2003) Characterization
of Pacific golden chanterelle (Cantharellus formosus) genet size
using co-dominant microsatellite markers. Mol Ecol 12:
1607–1618.
Edwards IP, Cripliver JL, Gillespie AR, Johnsen KH, Scholler M &
Turco RF (2004) Nitrogen availability alters macrofungal
community structure in optimally fertilized loblolly pine
forests. New Phytol 162: 755–770.
Ek H (1997) The influence of nitrogen fertilization on the carbon
economy of Paxillus involutus in ectomycorrhizal association
with Betula pendula. New Phytol 135: 133–142.
Ek H, Ahdersson S, Arnebrant K & Söderström B (1994) Growth
and assimilation of NH1
4 and NO3 by Paxillus involutus in
association with Betula pendula and Picea abies as affected by
substrate pH. New Phytol 128: 629–637.
Ericsson T, Rytter L & Vapaavouri E (1996) Physiology of carbon
allocation in trees. Biomass and Bioenergy 11: 115–127.
Erland S & Taylor AFS (2002) Diversity of ecto-mycorrhizal
fungal communities in relation to the abiotic environment.
Mycorrhizal Ecology (Ecological Studies Vol. 157) (van der
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
14
Heijden MGA & Sanders IR, eds), pp. 163–200. SpringerVerlag, Berlin.
Erland S, Söderström B & Andersson S (1990) Effects of liming
on ectomycorrhizal fungi infecting Pinus sylvestris L. II.
Growth rates in pure culture at different pH values compared
to growth rates in symbiosis with the host plant. New Phytol
115: 683–688.
Finlay RD & Read DJ (1986a) The structure and function of the
vegetative mycelium of ectomycorrhizal plants. I.
Translocation of 14C-labelled carbon between plants
interconnected by a common mycelium. New Phytol 103:
143–156.
Finlay RD & Read DJ (1986b) The structure and function of the
vegetative mycelium of ectomycorrhizal plants. II. The uptake
and distribution of phosphorus by mycelial strands
interconnecting host plants. New Phytol 103: 157–165.
Finlay RD, Ek H, Odham G & Söderström B (1988) Mycelial
uptake, translocation and assimilation of nitrogen from 15Nlabelled ammonium by Pinus sylvestris plants infected with
four different ectomycorrhizal fungi. New Phytol 110: 59–66.
Finlay RD, Ek H, Odham G & Söderström B (1989) Uptake,
translocation and assimilation of nitrogen from 15N-labelled
ammonium and nitrate sources by intact ectomycorrhizal
systems of Fagus sylvatica infected with Paxillus involutus. New
Phytol 113: 47–55.
Fiore-Donno A-M & Martin F (2001) Populations of
ectomycorrhizal Laccaria amethystina and Xerocomus spp.
show contrasting colonization patterns in a mixed forest. New
Phytol 152: 533–542.
Fransson PMA, Taylor AFS & Finlay RD (2000) Effects of
continuous optimal fertilization on belowground
ectomycorrhizal community structure in a Norway spruce
forest. Tree Physiol 20: 599–606.
Fransson PMA, Taylor AFS & Finlay RD (2001) Elevated
atmospheric CO2 alters root symbiont community structure in
forest trees. New Phytol 152: 431–442.
Fransson PMA, Taylor AFS & Finlay RD (2005) Mycelial
production, spread and root colonisation by the
ectomycorrhizal fungi Hebeloma crustuliniforme and Paxillus
involutus under elevated atmospheric CO2. Mycorrhiza 15:
25–31.
Gehring CA, Theimer TC, Whitham TG & Keim P (1998)
Ectomycorrhizal fungal community structure of pinyon pines
growing in two environmental extremes. Ecology 79:
1562–1572.
Genney DR, Anderson IC & Alexander IJ (2006) Fine-scale
distribution of pine ectomycorrhizas and their extramatrical
mycelium. New Phytol 170: 381–390.
Gherbi H, Delaruelle C, Selosse M-A & Martin F (1999) High
genetic diversity in a population of the ectomycorrhizal
basidiomycete Laccaria amethystina in a 150-year-old beech
forest. Mol Ecol 8: 2003–2013.
Giachini AJ, Souza LAB & Oliviera VL (2004) Species richness
and seasonal abundance of ectomycorrhizal fungi in
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
I.C. Anderson et al.
plantations of Eucalyptus dunnii and Pinus taeda in southern
Brazil. Mycorrhiza 14: 375–381.
Godbold DL & Berntson GM (1997) Elevated atmospheric CO2
concentration changes ectomycorrhizal morphotype
assemblages in Betula papyrifera. Tree Phytol 17: 347–350.
Godbold DL, Berntson GM & Bazzaz FA (1997) Growth and
mycorrhizal colonization of three North American tree species
under elevated atmospheric CO2. New Phytol 137: 433–440.
Godbold DL, Hoosbeek MR, Lukac M et al. (2006) Mycorrhizal
hyphal turnover as a dominant process for carbon input into
soil organic matter. Plant Soil 281: 15–24.
Goodman DM & Trofymow JA (1998) Distribution of
ectomycorrhizas in micro-habitats in mature and old growth
stands of Douglas fir on southeastern Vancouver Island. Soil
Biol Biochem 30: 2127–2138.
Göransson H, Wallander H, Ingerslev M & Rosengren U (2006)
Estimating the relative nutrient uptake from different soil
depths in Quercus robur, Fagus sylvatica and Picea abies. Plant
Soil 286: 87–97.
Gorissen A & Kuyper TW (2000) Fungal species-specific
responses of ectomycorrhizal Scots pine (Pinus sylvestris) to
elevated [CO2]. New Phytol 146: 163–168.
Griffiths RP, Castellano MA & Caldwell BA (1991) Hyphal mats
formed by two ectomycorrhizal fungi and their association
with Douglas-fir seedlings: a case study. Plant Soil 134:
255–259.
Grogan P, Baar J & Bruns TD (2000) Belowground
ectomycorrhizal community structure in a recently burned
bishop pine forest. J Ecol 88: 1051–1062.
Gryta H, Debaud J-C, Effosse A, Gay G & Marmeisse R (1997)
Fine-scale structure of populations of the ectomycorrhizal
fungus Hebeloma cylindrosporum in coastal sand dune forest
ecosystems. Mol Ecol 6: 353–364.
Gryta H, Carriconde F, Charcosset J-Y, Jargeat P & Gardes M
(2006) Population dynamics of the ectomycorrhizal fungal
species Tricholoma populinum and Tricholoma scalpturatum
associated with black poplar under differing environmental
conditions. Environ Microbiol 8: 773–786.
Guidot A, Debaud J-C & Marmeisse R (2001) Correspondence
between genet diversity and spatial distribution of above- and
belowground populations of the ectomycorrhizal fungus
Hebeloma cylindrosporum. Mol Ecol 10: 1121–1131.
Guidot A, Debaud J-C & Marmeisse R (2002) Spatial distribution
of the below-ground mycelia of an ectomycorrhizal fungus
inferred from specific quantification of its DNA in soil
samples. FEMS Microbiol Ecol 42: 477–486.
Guidot A, Debaud J-C, Effosse A & Marmeisse R (2004)
Belowground distribution and persistence of an
ectomycorrhizal fungus. New Phytol 161: 539–548.
Hagerberg D & Wallander H (2002) The impact of forest residue
removal and wood ash amendment on the growth of the
ectomycorrhizal external mycelium. FEMS Microbiol Ecol 39:
139–146.
Hagerberg D, Thelin G & Wallander H (2003) The production of
ectomycorrhizal mycelium in forests: relation between forest
FEMS Microbiol Rev ]] (2007) 1–19
15
Ectomycorrhizal fungal mycelia
nutrient status and local mineral sources. Plant Soil 252:
279–290.
Hagerberg D, Pallon J & Wallander H (2005) The elemental
content in the mycelium of the ectomycorrhizal fungus
Piloderma sp. during the colonization of hardened wood ash.
Mycorrhiza 15: 387–392.
Heinonsalo J, Hurme K-R & Sen R (2004) Recent 14C-labelled
assimilate allocation to Scots pine seedling root and
mycorrhizosphere compartments developed on reconstructed
podsol humus, E- and B- mineral horizons. Plant Soil 259:
111–121.
Hendricks JJ, Mitchell RJ, Kuehn KA, Pecot SD & Sims SE (2006)
Measuring external mycelia production of ectomycorrhizal
fungi in the field: the soil matrix matters. New Phytol 171:
179–186.
Hirose D, Kikuchi J, Kanzaki N & Futai K (2004) Genet
distribution of sporocarps and ectomycorrhizas of Suillus
pictus in a Japanese white pine plantation. New Phytol 164:
527–541.
Hitchcock CJ, Chambers SM & Cairney JWG (2006)
Development of polymorphic simple sequence repeat markers
for Pisolithus microcarpus. Mol Ecol Notes 6: 443–445.
Hobbie EA (2006) Carbon allocation to ectomycorrhizal fungi
correlates with belowground allocation in culture studies.
Ecology 87: 563–569.
Högberg MN & Högberg P (2002) Extramatrical ectomycorrhizal
mycelium contributes one-third of microbial biomass and
produces, together with associated roots, half the dissolved
organic carbon in a forest soil. New Phytol 154: 791–795.
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A,
Högberg MN, Nyberg G, Ottosson-Löfvenius M & Read DJ
(2001) Large-scale forest girdling shows that current
photosynthesis drives soil respiration. Nature 411: 789–792.
Horton TR & Bruns TD (2001) The molecular revolution in
ectomycorrhizal ecology: peeking into the black-box. Mol Ecol
10: 1855–1871.
Ineichen K, Wiemken V & Wiemken A (1995) Shoots, roots and
ectomycorrhiza formation of pine seedlings at elevated
atmospheric carbon dioxide. Plant Cell Environ 18: 703–707.
Izzo A, Agbowa J & Bruns TD (2005) Detection of plot-level
changes in ectomycorrhizal communities across years in an
old-growth mixed-conifer forest. New Phytol 166: 619–630.
Jacobson KM, Miller OK & Turner BJ (1993) Randomly
amplified polymorphic DNA markers are superior to somatic
incompatibility tests for discriminating genotypes in natural
populations of the ectomycorrhizal fungus Suillus granulatus.
Proc Natl Acad Sci USA 90: 9159–9163.
Jones MD, Durall DM & Cairney JWG (2003) Ectomycorrhizal
fungal communities in young forest stands regenerating after
clearcut logging. New Phytol 157: 399–422.
Kårén O & Nylund JE (1997) Effects of ammonium sulphate on
the community structure and biomass of ectomycorrhizal
fungi in a Norway spruce stand in southwestern Sweden. Can J
Bot 75: 1628–1642.
FEMS Microbiol Rev ]] (2007) 1–19
Kårén O, Högberg N, Dahlberg A, Grip K & Nylund J-E (1996)
Influence of drought on ectomycorrhizal species
composition morphotype versus PCR identification.
Mycorrhizas in Integrated Systems (Azcon-Aguilar C & Barea
JM, eds), pp. 43–46. European Commission, Brussels.
Kasurinen A, Keinanen MM, Kaipainen S, Nilsson LO,
Vapaavuori E, Kontro MH & Holopainen T (2005)
Belowground responses of silver birch trees exposed to
elevated CO2 and O-3 levels during three growing seasons.
Glob Change Biol 11: 1167–1179.
Kernaghan G & Harper KA (2001) Community structure of
ectomycorrhizal fungi across an alpine/subalpine ecotone.
Ecography 24: 181–188.
Kjller R (2006) Disproportionate abundance between
ectomycorrhizal root tips and their associated mycelia. FEMS
Microbiol Ecol 58: 214–224.
Klamer M, Roberts MS, Levine LH, Drake BG & Garland JL
(2002) Influence of elevated CO2 on the fungal community in
a coastal scrub oak forest soil investigated with terminal
restriction fragment length polymorphism analysis. Appl
Environ Microbiol 68: 4370–4376.
Koide RT, Xu B & Sharda J (2005a) Contrasting belowground
views of an ectomycorrhizal fungal community. New Phytol
166: 251–262.
Koide RT, Xu B, Sharda J, Lekberg Y & Ostiguy N (2005b)
Evidence of species interactions within an ectomycorrhizal
community. New Phytol 165: 305–316.
Korkama T, Fritze H, Pakkanen A & Pennanen T (2007)
Interactions between extraradical ectomycorrhizal mycelia,
microbes attached to mycelia and growth rate of Norway
spruce (Picea abies) clones. New Phytol 173: 798–807.
Kretzer AM, Dunham S, Molina R & Spatafora JW (2004)
Microsatellite markers reveal the below ground distribution of
genets of two species of Rhizopogon forming tuberculate
ectomycorrhizas on Douglas fir. New Phytol 161: 313–320.
Landeweert R, Hoffland E, Finlay RD, Kuyper TW & van
Breeman N (2001) Linking plants to rocks: ectomycorrhizal
fungi mobilize nutrients from minerals. Trends Ecol Evol 16:
248–254.
Landeweert R, Leeflang P, Kuyper TW, Hoffland E, Rosling A,
Wernars K & Smit E (2003a) Molecular identification of
ectomycorrhizal mycelium in soil horizons. Appl Environ
Microbiol 69: 327–333.
Landeweert R, Veenman C, Kuyper TW, Fritze H, Werners K &
Smit E (2003b) Quantification of ectomycorrhizal mycelium
in soil by real time PCR compared to conventional
quantification techniques. FEMS Microbiol Ecol 45: 283–292.
Landeweert R, Leeflang P, Smit E & Kuyper TW (2005) Diversity
of an ectomycorrhizal fungal community studied by a root tip
and total soil DNA approach. Mycorrhiza 15: 1–6.
Leake JR, Donnelly DP, Saunders EM, Boddy L & Read DJ (2001)
Rates and quantities of carbon flux to ectomycorrhizal
mycelium following 14C pulse labeling of Pinus sylvestris
seedlings: effects of litter patches and interaction with a wooddecomposer fungus. Tree Physiol 21: 71–82.
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
16
Leake JR, Donnelly DP & Boddy L (2002) Interactions between
ecto-mycorrhizal and saprotrophic fungi. Mycorrhizal Ecology
(Ecological Studies Vol. 157) (van der Heijden MGA & Sanders
IR, eds), pp. 345–372. Springer-Verlag, Berlin.
Lian C, Narimatsu M, Nara K & Hogetsu T (2006) Tricholoma
matsutake in a natural Pinus densiflora forest: correspondence
between above- and belowground genets, association with
multiple host trees and alteration of existing ectomycorrhizal
communities. New Phytol 171: 825–836.
Liang Y, Guo L & Ma K (2004) Genetic structure of a population
of the ectomycorrhizal fungus Russula vinosa in subtropical
woodlands in southwest China. Mycorrhiza 14: 235–240.
Liang Y, Guo L & Ma K (2005) Population genetic structure of an
ectomycorrhizal fungus Amanita manginiana in a subtropical
forest over two years. Mycorrhiza 15: 137–142.
Lilleskov EA & Bruns TD (2003) Root colonization dynamics of
two ectomycorrhizal fungi of contrasting life history strategies
are mediated by addition of organic nutrient patches. New
Phytol 159: 141–151.
Lilleskov EA, Fahey TJ, Horton TR & Lovett GM (2002)
Belowground ectomycorrhizal fungal community change over
a nitrogen deposition gradient in Alaska. Ecology 83: 104–115.
Lilleskov EA, Bruns TD, Horton TR, Taylor DL & Grogan P
(2004) Detection of forest stand-level spatial structure in
ectomycorrhizal fungal communities. FEMS Microbiol Ecol 49:
319–332.
Lindahl B (2000) Ectomycorrhizal fungi raid saprotrophic ones.
Mycol Res 104: 386–387.
Lindahl B, Stenlid J, Olsson S & Finlay RD (1999) Translocation
of 32P between interacting mycelia of a wood-decomposing
fungus and ectomycorrhizal fungi in microcosm systems. New
Phytol 144: 183–193.
Lindahl B, Stenlid J & Finlay RD (2001) Effects of resource
availability on mycelial interactions and 32P transfer between a
saprotrophic and an ectomycorrhizal fungus in soil
microcosms. FEMS Microbiol Ecol 38: 43–52.
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P,
Stenlid J & Finlay RD (2007) Spatial separation of litter
decomposition and mycorrhizal nitrogen uptake in a boreal
forest. New Phytol 173: 611–620.
Mahmood S, Finlay RD, Erland S & Wallander H (2001)
Solubilisation and colonisation of wood ash by
ectomycorrhizal fungi isolated from a wood ash fertilised
spruce plot. FEMS Microbiol Ecol 35: 151–161.
Markkola AM, Ahonen-Jonnarth U, Roitta M, Strommer R &
Hyvarinen M (2002) Shift in ectomycorrhizal community
structure in Scots pine (Pinus sylvestris L.) seedling roots as a
response to nickel deposition and removal of lichen cover.
Environ Pollut 120: 797–803.
Massicotte HB, Molina R, Tackaberry LE, Smith JE &
Amaranthus MP (1999) Diversity and host specificity of
ectomycorrhizal fungi retrieved from three adjacent forest sites
by five host species. Can J Bot 77: 1053–1076.
Moser A, Peterson CA, D’Allura JA & Southworth D (2005)
Comparison of ectomycorrhizas of Quercus garryana
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
I.C. Anderson et al.
(Fagaceae) on serpentine and non-serpentine soils in
southwestern Oregon. Am J Bot 92: 224–230.
Mueller RC & Gehring CA (2006) Interactions between an aboveground plant parasite and belowground ectomycorrhizal
fungal communities on pinyon pine. J Ecol 94: 276–284.
Murata H, Ohta A, Yamada A, Narimatsu M & Futamura N
(2005) Genetic mosaics in the massive persisting rhizosphere
colony ‘‘shiro’’ of the ectomycorrhizal basidiomycete
Tricholoma matsutake. Mycorrhiza 15: 505–512.
Nilsson LO (2004) External mycelia of mycorrhizal fungi –
responses to elevated N in forest ecosystems. PhD thesis, Lund
University, Lund.
Nilsson LO & Wallander H (2003) Production of external
mycelium by ectomycorrhizal fungi in a Norway spruce forest
was reduced in response to nitrogen fertilization. New Phytol
158: 409–416.
Nilsson LO, Giesler R, Bååth E & Wallander H (2005) Growth and
biomass of mycorrhizal mycelia in coniferous forests along
short natural nutrient gradients. New Phytol 165: 613–622.
Norby RJ, O’Neill EG, Hood WG & Luxmore RJ (1987) Carbon
allocation, root exudation and mycorrhizal colonization of
Pinus echinata seedlings grown under CO2 enrichment. Tree
Physiol 3: 203–210.
Ogawa M (1977) Microbial ecology of mycorrhizal fungus –
Tricholoma matsutake (Ito et Imai) Sing. in pine forest. IV. The
shiro of T. matsutake in the fungal community. Bull Gov Forest
Exp Station 297: 59–104.
Palfner G, Casanova-Katny MA & Read DJ (2005) The
mycorrhizal community in a forest chronosequence of Sitka
spruce [Picea sitchensis (Bong.) Carr.] in Northern England.
Mycorrhiza 15: 571–579.
Pendall E, Bridgham S, Hanson PJ et al. (2004) Belowground
process responses to elevated CO2 and temperature: a
discussion of observations, measurement methods, and
models. New Phytol 162: 311–322.
Pérez-Moreno J & Read DJ (2000) Mobilization and transfer of
nutrients from litter to tree seedlings via the vegetative
mycelium of ectomycorrhizal plants. New Phytol 145: 301–309.
Pérez-Moreno J & Read DJ (2001a) Exploitation of pollen by
mycorrhizal mycelial systems with special reference to nutrient
recycling in boreal forests. Proc Roy Soc Lond B Bio 268:
1329–1335.
Pérez-Moreno J & Read DJ (2001b) Nutrient transfer from soil
nematodes to plants: a direct pathway provided by the
mycorrhizal mycelial network. Plant Cell Environ 24:
1219–1226.
Peter M (2006) Ectomycorrhizal fungi – fairy rings and the woodwide web. New Phytol 171: 685–687.
Peter M, Ayer F & Egli S (2001) Nitrogen addition in a Norway
spruce stand altered macromycete sporocarp production and
belowground ectomycorrhizal species composition. New
Phytol 149: 311–325.
Peterson RL, Massicotte HB & Melville LH (2004) Mycorrhizas:
Anatomy and Cell Biology. CABI Publishing, Wallingford, UK.
FEMS Microbiol Rev ]] (2007) 1–19
17
Ectomycorrhizal fungal mycelia
Qian XM, Kottke I & Oberwinkler F (1998) Influence of liming
and acidification on the activity of the mycorrhizal
communities in a Picea abies (L.) Karst. stand. Plant Soil 199:
99–109.
Raidl S, Bonfigli R & Agerer R (2005) Calibration of quantitative
real time PCR by correlation with hyphal biomass and ITS
copies in mycelia of Piloderma croceum. Plant Biol 7: 713–717.
Rapp C & Jentschke G (1994) Acid deposition and
ectomycorrhizal symbiosis: field investigations and causal
relationships. Effects of Acid Rain on Forest Processes (Godbold
DL & Hütterman A, eds), pp. 183–230. Wiley-Liss Inc, New
York.
Rayner ADM (1991) The challenge of the individualistic
mycelium. Mycologia 83: 48–71.
Read DJ (1992) The mycorrhizal mycelium. Mycorrhizal
Functioning: An Integrative Plant-Fungal Process (Allen MJ,
ed), pp. 102–133. Chapman, Hall, New York.
Read DJ & Perez-Moreno J (2003) Mycorrhizas and nutrient
cycling in ecosystems – a journey towards relevance? New
Phytol 157: 475–492.
Read DJ, Leake JR & Perez-Moreno J (2004) Mycorrhizal fungi as
drivers of ecosystem processes in heathland and boreal forest
biomes. Can J Bot 82: 1243–1263.
Redecker D, Szaro TM, Bowman RJ & Bruns TD (2001) Small
genets of Lactarius xanthogalactus, Russula cremoricolor and
Amanita francheti in late-stage ectomycorrhizal successions.
Mol Ecol 10: 1025–1034.
Rey A & Jarvis PG (1997) Growth responses of young birch trees
(Betula pendula Roth.) after four and a half years of CO2
exposure. Ann Bot 80: 809–816.
Rillig MC, Treseder KK & Allen MF (2002) Global change and
mycorrhizal fungi. Mycorrhizal Ecology (Ecological Studies Vol.
157) (van der Heijden MGA & Sanders IR, eds), pp. 135–160.
Springer-Verlag, Berlin.
Riviere T, Natarajan K & Dreyfus B (2006) Spatial distribution of
ectomycorrhizal Basidiomycete Russula subsect. Foetentinae
populations in a primary dipterocarp rainforest. Mycorrhiza
16: 143–148.
Robertson SJ, Tackaberry LE, Egger KN & Massicotte HB (2006)
Ectomycorrhizal fungal communities of black spruce differ
between wetland and upland forests. Can J For Res 36:
972–985.
Rosling A, Landeweert R, Lindahl BD, Larsson K-H, Kuyper TW,
Taylor AFS & Finlay RD (2003) Vertical distribution of
ectomycorrhizal fungal taxa in a podsol soil profile. New Phytol
159: 775–783.
Rosling A, Lindahl BD & Finlay RD (2004) Carbon allocation to
ectomycorrhizal roots and mycelium colonising different
mineral substrates. New Phytol 162: 795–802.
Roth DR & Fahey TJ (1998) The effects of acid precipitation and
ozone on the ectomycorrhizae of red spruce saplings. Water
Air Soil Pollut 103: 263–276.
Rouhier H & Read DJ (1998) Plant and fungal responses to
elevated atmospheric carbon dioxide in mycorrhizal seedlings
of Pinus sylvestris. Environ Exp Bot 40: 237–246.
FEMS Microbiol Rev ]] (2007) 1–19
Rouhier H & Read DJ (1999) Plant and fungal responses to
elevated atmospheric CO2 in mycorrhizal seedlings of Betula
pendula. Environ Exp Bot 42: 231–241.
Rousseau JVD, Sylvia DM & Fox AJ (1994) Contribution of
ectomycorrhiza to the potential nutrient-absorbing surface of
pine. New Phytol 128: 639–644.
Rygiewicz PT, Martin KJ & Tuininga AR (2000) Morphotype
community structure of ectomycorrhizas on Douglas fir
(Pseudotsuga menziesii Mirb. Franco) seedlings grown under
elevated atmospheric CO2 and temperature. Oecologia 124:
299–308.
Sawyer NA, Chambers SM & Cairney JWG (1999) Molecular
investigation of genet distribution and genetic variation of
Cortinarius rotundisporus. New Phytol 142: 561–568.
Sawyer NA, Chambers SM & Cairney JWG (2001) Distribution
and persistence of Amanita muscaria genotypes in Australian
Pinus radiata plantations. Mycol Res 105: 966–970.
Sawyer NA, Chambers SM & Cairney JWG (2003) Distribution of
Amanita spp. genotypes under eastern Australian sclerophyll
vegetation. Mycol Res 107: 1157–1163.
Schneider K, Renker C & Maraun M (2005) Orbatid mite (Acari,
Orbatida) feeding on ectomycorrhizal fungi. Mycorrhiza 16:
67–72.
Schubert R, Raidl S, Funk R, Bahnweg G, Müller-Starck G &
Agerer R (2003) Quantitative detection of agar-cultivated and
rhizotron-grown Piloderma croceum Erikss. & Hjortst. by
ITS1-based fluorescent PCR. Mycorrhiza 13: 159–165.
Selosse M-A (2003) Founder effect in a young Leccinum
duriusculum (Schultzer) Singer population. Mycorrhiza 13:
143–149.
Selosse M-A, Jacquot D, Bouchard D, Martin F & Le Tacon F
(1998) Temporal persistence and spatial distribution of an
American inoculant strain of the ectomycorrhizal
basidiomycete Laccaria bicolor in a French forest plantation.
Mol Ecol 7: 561–573.
Selosse M-A, Martin F & Le Tacon F (2001) Intraspecific
variation in fruiting phenology in an ectomycorrhizal Laccaria
population under Douglas fir. Mycol Res 105: 524–531.
Selosse M-A, Richard F, He X & Simard SW (2006) Mycorhizal
networks: des liaisons dengereuses? Trends Ecol Evol 21:
621–628.
Sen R (1990) Intraspecific variation in two species of Suillus from
Scots pine (Pinus sylvestris L.) forests based on somatic
incompatibility and isozyme analyses. New Phytol 114:
607–616.
Sen R (2000) Budgeting for the wood-wide web. New Phytol 145:
161–165.
Shi LB, Guttenberger M, Kottke I & Hampp R (2002) The effect
of drought on mycorrhizas of beech (Fagus sylvatica L.):
changes in community structure, and the content of
carbohydrates and nitrogen storage bodies of the fungi.
Mycorrhiza 12: 303–311.
Simard SW & Durall DM (2004) Mycorrhizal networks: a review
of their extent, function, and importance. Can J Bot 82:
1140–1165.
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
18
Smit E, Veenman C & Baar J (2003) Molecular analysis of
ectomycorrhizal basidiomycete communities in a Pinus
sylvestris L. stand reveals long-term increased diversity after
removal of litter and humus layers. FEMS Microbiol Ecol 45:
49–57.
Smith JE, McKay D, Brenner G, McIver J & Spatafora J (2005)
Early impacts of forest restoration treatments in the
ectomycorrhizal fungal community and fine root biomass in a
mixed conifer forest. J Appl Ecol 42: 526–535.
Smith SE & Read DJ (1997) Mycorrhizal Symbiosis. Academic
Press, London.
Söderström B (1992) The ecological potential of the
ectomycorrhizal mycelium. Mycorrhiza in Ecosystems
(Alexander IJ, Fitter AH, Lewis DH & Read DJ, eds), pp.
77–83. CAB International, London.
Stendell ER, Horton TR & Bruns TD (1999) Early effects of
prescribed fire on the structure of the ectomycorrhizal fungus
community in a Sierra Nevada ponderosa pine forest. Mycol
Res 103: 1353–1359.
Taylor AFS (2002) Fungal diversity in ectomycorrhizal
communities: sampling effort and species detection. Plant Soil
244: 19–28.
Taylor AFS & Alexander IJ (1989) Demography and population
dynamics of ectomycorrhizas of Sitka spruce fertilized with N.
Agr Ecosyst Environ 28: 493–496.
Taylor AFS, Martin F & Read DJ (2000) Fungal diversity in
ectomycorrhizal communities of Norway spruce (Picea abies
[L.] Karst.) and beech (Fagus sylvatica L.) along north-south
transects in Europe. Carbon and Nitrogen Cycling in European
Forest Ecosystems (Schultze E-D, ed), pp. 343–365. Springer
Verlag, Heidelberg.
Tedersoo L, Kõljalg U, Hallenberg N & Larsson K-H (2003) Fine
scale distribution of ectomycorrhizal fungi and roots across
substrate layers including coarse woody debris in a mixed
forest. New Phytol 159: 153–165.
Tedersoo L, Hansen K, Perry BA & Kjller R (2006) Molecular
and morphological diversity of pezizalean ectomycorrhiza.
New Phytol 170: 581–596.
Thompson W (1984) Distribution, development and functioning
of mycelial cord systems of decomposer basidiomycetes of the
deciduous woodland floor. The Ecology and Physiology of the
Fungal Mycelium (Jennings DH & Rayner ADM, eds), pp.
185–214. Cambridge University Press, Cambridge.
Timonen S & Sen R (1998) Heterogeneity of fungal and plant
enzyme expression in intact Scots pine – Suillus bovinus and Paxillus involutus mycorrhizospheres developed in natural
forest humus. New Phytol 138: 355–366.
Timonen S, Finlay RD, Olsson S & Söderström B (1996)
Dynamics of phosphorus translocation in intact
ectomycorrhizal systems: non-destructive monitoring using a
b-scanner. FEMS Microbiol Ecol 19: 171–180.
Tingey DT, Phillips DL, Johnson MG, Strom MJ & Ball JT (1997)
Effects of elevated CO2 and N fertilization on fine root
dynamics and fungal growth in seedling Pinus ponderosa.
Environ Exp Bot 37: 73–83.
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c
I.C. Anderson et al.
Toljander JF, Eberhardt U, Toljander YK, Paul LR & Taylor AFS
(2006) Species composition of an ectomycorrhizal fungal
community along a local nutrient gradient in a boreal forest.
New Phytol 170: 873–883.
Visser S (1995) Ectomycorrhizal fungal succession in jack pine
stands following wildfire. New Phytol 129: 389–401.
Vrålstad T, Myhre E & Schumacher T (2002) Molecular diversity
and phylogenetic affinities of symbiotic root-associated
ascomycetes of the Helotiales in burnt and metal polluted
habitats. New Phytol 155: 131–148.
Walker JF, Miller OK & Horton JL (2005) Hyperdiversity of
ectomycorrhizal fungus assemblages on oak seedlings in mixed
forests in the southern Appalachian Mountains. Mol Ecol 14:
829–838.
Walker RF, Johnson DW, Geisinger DR & Ball JT (1998) Growth
and ectomycorrhizal colonization of ponderosa pine seedlings
supplied with different levels of atmospheric CO2 and soil N
and P. Forest Ecol Manag 109: 9–20.
Wallander H (2006) External mycorrhizal mycelia – the
importance of quantification in natural ecosystems. New
Phytol 171: 240–242.
Wallander H & Pallon J (2005) Temporal changes in the elemental
composition of Rhizopogon rhizomorphs during colonization
of patches with fresh organic matter or acid-washed sand.
Mycologia 97: 295–303.
Wallander H, Nilsson L-O, Hagerberg D & Bååth E (2001)
Estimation of the biomass and seasonal growth of external
mycelium of ectomycorrhizal fungi in the field. New Phytol
151: 753–760.
Wallander H, Johansson L & Pallon J (2002) PIXE analysis to
estimate the elemental composition of ectomycorrhizal
rhizomorphs grown in contact with different minerals in forest
soil. FEMS Microbiol Ecol 39: 147–156.
Wallander H, Mahmood S, Hagerberg D, Johansson L & Pallon J
(2003) Elemental composition of ectomycorrhizal mycelia
identified by PCR-RFLP analysis and growth in contact with
apatite or wood ash in forest soil. FEMS Microbiol Ecol 44:
57–65.
Wallander H, Göransson H & Rosengren U (2004) Production,
standing biomass and natural abundance of 15N and 13C in
ectomycorrhizal mycelia collected at different soil depths in
two forest types. Oecologia 139: 89–97.
Wright DP, Johansson T, Le Quéré A, Söderström B & Tunlid A
(2005) Spatial patterns of gene expression in the extramatrical
mycelium and mycorrhizal root tips formed by the
ectomycorrhizal fungus Paxillus involutus in association with
birch (Betula pendula) seedlings in soil microcosms. New
Phytol 167: 579–596.
Wu B, Nara K & Hogetsu T (1999) Competition between
ectomycorrhizal fungi colonizing Pinus densifolia. Mycorrhiza
9: 151–159.
Wu B, Nara K & Hogetsu T (2002) Spatiotemporal transfer of
carbon-14-labelled photosynthate from ectomycorrhizal Pinus
densiflora seedlings to extramatrical mycelia. Mycorrhiza 12:
83–88.
FEMS Microbiol Rev ]] (2007) 1–19
19
Ectomycorrhizal fungal mycelia
Wu B, Nara K & Hogetsu T (2005) Genetic structure of
Cenococcum geophilum populations in primary successional
volcanic deserts on Mount Fuji as revealed by microsatellite
markers. New Phytol 165: 285–293.
Wurzenburger N, Hartshorn AS & Hendrick RL (2004)
Ectomycorrhizal fungal community structure across a bogforest ecotone in southeastern Alaska. Mycorrhiza 14: 383–389.
Zhou Z, Miwa M & Hogetsu T (2000) Genet distribution of
ectomycorrhizal fungus Suillus grevillei populations in two
FEMS Microbiol Rev ]] (2007) 1–19
Larix kaempferi stands over two years. J Plant Res 113:
365–374.
Zhou Z, Miwa M & Hogetsu T (2001a) Polymorphism of simple
sequence repeats reveals gene flow within and between
ectomycorrhizal Suillus grevillei populations. New Phytol 149:
339–348.
Zhou Z, Miwa M, Matsuda Y & Hogetsu T (2001b) Spatial
distribution of the subterranean mycelia and ectomycorrhizae
of Suillus grevillei genets. J Plant Res 114: 179–185.
2007 Federation of European Microbiological Societies
Published by Blackwell Publishing Ltd. All rights reserved
c