Normal sympathetic nervous system response in reflex sympathetic
advertisement

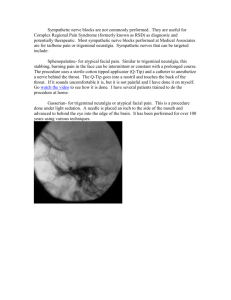
Normal sympathetic nervous system response in reflex sympathetic dystrophy María de Lourdes Figuerola*,*** Gloria Levin* Alicia Bertotti** Jorge Ferreiro*** Marta Barontini* * CEDIE, CONICET, Ricardo Gutiérrez Children’s Hospital; ** Service of Neurophysiology; *** Service of Neurology, José de San Martín Hospital, Buenos Aires, Argentina Reprint requests to: Prof. María de Lourdes Figuerola Centro de Investigaciones Endocrinológicas Hospital de Niños Ricardo Gutiérrez Gallo 1360 (1425) Buenos Aires, Argentina E-mail: mfiguerola@intramed.net.ar Accepted for publication: May 8, 2002 Summary We evaluated sympathetic nervous system activity by sympathetic skin response (SSR) recording and we further investigated sympathetic and opioid outflow indirectly in patients with features of reflex sympathetic dystrophy by measuring concentrations of plasma catecholamines (CAs) and their metabolites and plasma metenkephalin (ME), before and after corticoid treatment. Six patients were studied. Basal SSR latencies, morphologies and amplitudes were normal in five patients. In one woman, latency and amplitude were also normal but the morphology was disturbed. Basal plasma ME, CA and metabolite levels were similar in the affected and non-affected limbs and a significant increase in plasma ME concentrations was observed in both affected and nonaffected limbs after two weeks of steroid treatment. Altogether these results point to an adaptive supersensitivity rather than a sympathetic hyperactivity in this syndrome; also, they indicate that the therapeutic effect of steroids adds, to their known anti-inflammatory action, a stimulatory action on the endogenous opioid system. KEY WORDS: Catecholamines, complex regional pain syndrome, metenkephalin, reflex sympathetic dystrophy, sympathetic skin response. Introduction Reflex sympathetic dystrophy (RSD) is a complication occurring even after minor injury to, or operation on, a limb. RSD is a descriptive diagnostic term that implicates the sympathetic nervous system in a reflex response to injury, with consequent dystrophic changes. Functional Neurology 2002; 17(2): 77-81 The pathophysiology of RSD remains unknown. In a revised taxonomic system of RSD, disorders previously considered to be RSD or causalgia were classified under the term complex regional pain syndrome (CRPS), which is based entirely on clinical criteria. The criteria for inclusion under this general term CRPS are the presence of regional pain – spontaneous and/or evoked – and other sensory changes following a noxious event. There are two types of CRPS, I and II, corresponding to RDS and causalgia respectively (1). It has been hypothesized that if injury to a limb normally provokes a rise in opioid-based modulation of activity in the regional sympathetic ganglia, it is possible that failure of this process occurs in some susceptible individuals, leading to localized signs of opioid withdrawal (2). The pain is associated with changes in skin color and temperature, abnormal sweating, edema and sometimes motor abnormalities. Hence, the sympathetic skin response (SSR) has been postulated as a diagnostic test (3). We evaluated sympathetic nervous system activity by SSR recording and we further investigated sympathetic and opioid outflow indirectly in patients with type I CRPS features, by measuring concentrations of plasma catecholamines (CAs) and their metabolites, and plasma met-enkephalin (ME). Venous blood was taken from the painful and normal limbs before and after steroid treatment. Materials and methods Six patients, four males and two females aged 26 to 76, with clinical findings for type I CRPS were studied. Diagnosis was made according to the consensus of the International Association for the Study of Pain. All the patients had an upper limb affected (right side in 4 and left side in 2). The onset of the syndrome was preceded by a noxious event involving the limb – distortion (3 cases), bone fracture (2 cases) and skin lesions (1 case) – occurring 2 weeks to 4 months beforehand. Clinical characteristics of patients are shown in Table I (see over). All 6 patients were tested for SSR. To obtain SSRs, superficial electrodes were placed on the palm (active) and referenced to the dorsum of the hand (indifferent). The ground electrode was proximal to the recording electrodes. Simultaneous bilateral recordings from the upper limbs were made (4). The recording time was 10 sec, the lower limit 0.5 Hz, and the upper limit 2000 Hz. Electrical stimulation was provoked with a constant current stimulus (0.2 msec, supramaximal, usually 10-30 mAmp). The stimulus was strong but well tolerated. It was applied at irregular intervals and at an approximate frequency of 1/min to avoid habituation. Patients were relaxed and free from any acoustic disturbances or time pressure. Light was dim. Room temperature was com- 77 M. Figuerola et al. Table I - Clinical characteristics of CRPS type I patients Patient Sex Age Initial Injury Duration (mths) Pain Temperature difference Hyperhydrosis Edema 1 M 26 upper limb distortion 0.5 yes yes yes yes 2 M 35 elbow fracture 1.6 yes yes yes no 3 F 52 shoulder fracture 4 yes yes no yes 4 M 48 upper limb distortion 3 yes no yes yes 5 M 76 skin injury 3.4 yes yes yes yes 6 F 41 upper limb distortion 4 yes yes yes yes fortable and skin surface temperature was 32°C. Latencies from the palm ranging from 1.3 to 1.5 msec and amplitudes > 200 µV were considered normal. Blood samples for simultaneous plasma ME and CA measurement were taken from both upper limbs with subjects resting in supine position. We used an indwelling intravenous needle inserted 30 minutes before sampling. All subjects were tested between 9 and 10 a.m., before and after two weeks of treatment with 1mg/kg/day prednisone. Blood for ME measurements was put into polypropylene tubes kept on ice containing 10 mg/ml of a protease inhibitor cocktail (10,000 IUC/ml aprotinin, 0.23 mM citric acid, and 0.024 mM EDTA). Blood was centrifuged at 4°C and plasma was immediately stored at 20°C. ME was extracted from plasma by adsorption chromatography in Amberlite XAD-2 columns. ME was measured by radioimmunoassay using specific antiserum (5). Under these experimental conditions, ME immunoreactivity corresponded to the free ME molecule as evidenced by reverse-phase high pressure liquid chromatography (RP-HPLC) (5). Iodinated ME was used as a tracer. Antiserum for ME showed a 100% cross-reactivity with met-(O)-enkephalin, 0.3% with leucine-enkephalin and less than 0.01% with leuenkephalin-arginine, dynorphin and endorphins (6). Blood for free plasma CA (adrenaline (A), noradrenaline (NA), dopamine (DA)) and dihydroxyphenylglycol (DHPG), dihydroxyphenylalanine (DOPA) and dihydroxyphenylacetic acid (DOPAC) measurements was poured into plastic tubes kept on ice containing 5 µl heparin/ml whole blood. Plasma was immediately separated by centrifugation at 2000 g for 20 min at 4°C and kept at -70°C until processed. Catechols in 1000 µl aliquots of plasma were partially purified by batch alumina extraction, and separated by RP-HPLC using a 4.6 x 250 mm Zorbax R xC18 column (New England Nuclear, Du Pont, Boston, MA, USA). Quantifications were made by current produced upon exposure of the column effluent to oxidizing and then reducing potentials in series using a triple-electrode system (Coulochem II, ESA, Bedford, MA, USA) (7). Recovery through the alumina extraction step averaged 70-80% for CAs and 45-55% for DHPG. Catechol concentrations in each sample were corrected for recovery of a dihydroxybenzylamine internal standard. Levels of DHPG, DOPA and DOPAC were further corrected for dif- 78 ferences in recovery of the internal standard and this catechol in a mixture of external standard. Written consent was obtained from all patients. The significance of the differences found in patient plasma levels from both sides before and after prednisone treatment was analyzed by T test for paired samples. Data were expressed as mean ± SEM. Results Table II shows data from the SSR studies. Basal SSR latencies, morphologies and amplitudes were normal in five patients. In one woman (patient no. 6), amplitude was lower but still within the normal range. Latency was also normal but the morphology was disturbed. Figure 1 shows the recording from a representative patient with normal SSR parameters and from patient 6. Plasma ME concentrations in basal conditions in both limbs are shown in Figure 2. Basal plasma ME levels were similar for the affected and non-affected limbs: 0.21±0.01 and 0.24±0.01 pmol/ml respectively. In spite of a tendency to lower plasma A and DOPAC concentrations in the basal samples from the affected limb, no significant differences could be observed in plasma levels of DOPA, CA, DHPG (the intraneuronal metabolite of NA), and DOPAC (the metabolite of DA) between the two limbs explored (Table III). After 2 weeks of prednisone treatment all patients im- Table II - Individual SSR responses obtained after median nerve stimulation on the affected side. Patient Latency msec Amplitude µV Morphology 1 1.45 1540 normal 2 1.38 2500 normal 3 1.48 750 normal 4 1.33 1700 normal 5 1.40 3410 normal 6 1.33 520 abnormal Functional Neurology 2002; 17(2): 77-81 Normal SNS response in reflex sympathetic dystrophy A significant increase in plasma ME concentrations was observed in both affected and non-affected limbs after two weeks of prednisone treatment: 0.33±0.02 and 0.34±0.02 pmol/ml respectively (p<0.003 vs basal values) (Fig. 2). Discussion Fig. 1 - SSR response. Upper panel: normal bilateral SSR obtained after median nerve stimulation in a representative patient. Lower panel: upper line represents the SSR obtained from the affected limb (normal latency and amplitude; abnormal configuration). Lower line represents the normal SSR obtained from the unaffected limb (patient no. 6). proved clinically. Clinical improvement was established by a subjective improvement of spontaneous pain and lack of painful response to mechanical stimuli. No differences were found in plasma CA levels after prednisone treatment (Table III). Five of the six patients had normal values of plasma DA (normal range: 0-200 pg/ml) and one patient presented increased levels of plasma DA in the unaffected limb, before and after prednisone treatment. This study evaluates SSR, opioid and sympathetic activity simultaneously in CRPS I. The pathogenesis of CRPS I is not yet understood, and diagnosing and treating patients is difficult. SSR has been advocated as a simple means of assessing sympathetic sudomotor outflow in central and peripheral nervous system disorders. SSR is a change in skin potential following arousal stimulation, first described by Tarchanoff (8). It is a polysynaptic reflex that is activated by a variety of afferent inputs (9). The final efferent pathway involves pre- and post-ganglionic sympathetic sudomotor fibers and ultimately activation of sweat glands by the sympathetic outflow. The reflex is coordinated in the posterior hypothalamus, upper brain stem reticular formation and spinal cord. The responses are generated in deep layers of the skin by reflex activation of sweat glands via cholinergic sudomotor sympathetic efferent fibers. Because the reflex is multisynaptic, latency, amplitude, wave form and tendency to habituation are variable. The potentials may be mono, bi or triphasic (4,9). It has been reported that in RSD, the mean amplitude of SSR in the involved limb is greater than in the uninvolved limb, and onset latency of SSR in the involved limb is shorter than that of the uninvolved limb (9). Conversely, we found normal responses in 5/6 evaluated patients. The only patient (number 6) who presented the abnormal SSR response exhibited no other peculiarity. However, as in other generalized abnormalities of sympathetic sudomotor dysfunction, autonomic dysfunction or small fiber neuropathy, the correlation of the abnormal SSR is often poor. There is no close correlation between the presence or the absence of SSR and the severity of the autonomic dysfunction. So the results are not conclusive. Early features of CRPS I in a limb (inappropriate pain, altered cutaneous sensation, excessive sweating, periph- Fig. 2 - Plasma ME levels in basal conditions and after prednisone treatment in both limbs. Functional Neurology 2002; 17(2): 77-81 79 M. Figuerola et al. Table III - Plasma levels of CA, DOPA, DOPAC and DHPG in the two limbs explored Affected Limb Non Affected Limb Pre Treatment Post Treatment 37±9 41±10 64±13 70±19 NA pg/ml 246±67 221±60 235±57 259±48 DA pg/ml 168±54 86±20 278±162 366±174 DOPA pg/ml 936±223 1072±283 896±159 898±239 1907±311 2108±191 4829±1136 3141±295 992±119 1100±157 867±135 1208±166 A pg/ml DOPAC pg/ml DHPG pg/ml Pre Treatment Post Treatment Abbreviations: A = adrenaline; NA = noradrenaline; DA = dopamine; DOPA = dihydroxyphenylalanine; DOPAC = dihydroxyphenylacetic acid; DHPG = dihydroxyphenylglycol. eral vascular instability and tremor) resemble the general effects of autonomic arousal associated with opioid withdrawal. Injury to a limb normally provokes a rise in opioid-based modulation of activity in regional sympathetic ganglia. It is possible that failure of this process occurs in some susceptible individuals, leading to localized signs of opioid withdrawal (2). The site of action of the opioids within the ganglion would most probably be on the presynaptic terminals of preganglionic nerve fibers (10). Increased blood concentrations of opioids are known to occur during strenuous muscular exercise (11). This effect might be mirrored by decreased opioid activity within the stellate and lumbar sympathetic ganglia when the adjacent limb is immobilized. Considering a failure of the opioid-based modulation of activity in regional sympathetic ganglia as responsible, at least in part, for the syndrome, it is possible that the common practice of immobilization of minor limb injuries could contribute to the abolition of regional opioid modulation, by reducing neural traffic between limb and spinal cord (2). However, basal plasma ME levels in our patients were within our control range and we did not find any differences when comparing painful and nonpainful sides. The importance of the sympathetic nervous system in the generation of pain has been the focus of a long, if controversial, debate (12). Sympatholytic therapy can abolish pain and hyperalgesia, and in CRPS patients an intracutaneous injection of adrenoceptor agonists rekindled pain and hyperalgesia. An explanation consistent with these findings is that primary afferents acquire a sensitivity to CAs, which in some conditions extends to the receptive terminals innervating a symptomatic skin region. On the other hand it has been assumed that an increase in sympathetic outflow causes sweating and changes in peripheral blood flow in CRPS I (13). However multiunit-microelectrode recordings of sympathetic outflow to affected skin failed to confirm that the sympathetic outflow is increased. Furthermore, the vasoconstrictor tone was found to be reduced rather than increased in the affected limb. Since CAs and ME are released from the periphery (i.e., sympa- 80 thetic ganglia), levels should be similar on both sides of the body if both limbs are kept in similar environments. Different plasma concentrations in the painful and normal limbs would indicate that the local clearance rate differed on the two sides. Our results show a tendency of adrenaline and DOPAC to be lower in the painful limb, so this entity appears to be associated with reduced rather than increased sympathetic outflow. We have no explanation for the higher plasma DA levels found in one patient in the unaffected limb. No differences in plasma A concentrations between affected and unaffected limbs have been reported. Besides, plasma NA and DHPG levels have been found to be lower on the painful side. These findings, like ours, do not support the view that autonomic disturbances in CRPS are due to sympathetic overactivity, but are more consistent with an adaptive supersensitivity of the SNS (13). Sudeck in 1902 first attributed an inflammatory pathogenesis to CRPS I. The idea of an inflammatory pathogenesis is based on the observation that in the acute phase all classical signs and symptoms of inflammation are present. Moreover, the therapeutic effect of corticosteroids in some patients supports this idea (14). In addition, scintigraphic investigations with marked immunoglobulins have shown an intraosseous plasma ex travasation in patients with CRPS I, which supports an inflammatory component of the disorder (15). It is difficult to explain the whole syndrome as exclusively peripheral. Lesioned afferent axons may generate spontaneous and evoked ectopic impulses, so the decoding of both nociceptive and non-nociceptive afferent information exerted by supraspinal systems is changed, resulting in distorted information processing in CNS. All our patients were treated with 1mg/kg/day prednisone and their symptoms improved. After two weeks they were free from pain. At that moment an increase in plasma ME concentration in both limbs was observed, pointing to a general effect of steroid action. Previously, we had found an increase in plasma ME levels after Functional Neurology 2002; 17(2): 77-81 Normal SNS response in reflex sympathetic dystrophy prednisone treatment in patients with cluster headache (16). Altogether, the results from this study seem to indicate an adaptive supersensitivity rather than a sympathetic hyperactivity in this syndrome. The therapeutic effect of steroids adds to their known anti-inflammatory action a stimulatory action on the endogenous opioid system. Acknowledgments Fundación René Barón kindly supported style correction of this paper. Drs Barontini and Levin and Prof. Figuerola are senior researchers from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) Argentina. References 11. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definition of Terms, Marskey H, Bogduk N eds 2nd Edition. Seattle; IASP Press 1994 12. Hannington-Kiff JG. Does failed natural opioid modulation in regional sympathetic ganglia cause reflex sympathetic dystrophy? Lancet 1991,338:1125-1127 13. Chelimsky T, Low P, Naessens J, Wilson P, Amadio P, O’Brien P. Value of autonomic testing in reflex sympathetic dystrophy. Mayo Clin Proc 1995; 70:1029-1040 14. Claus D, Schondorf R. Sympathetic skin response. In: Deuschl J, Eisen A eds Recommendations for the Practice of Clinical Neurophysiology: Guidelines of the International Federation of Clinical Neurophysiology. Suppl 52 to EEG and Clinical Neurophysiology. Elsevier 1999:277-282 15. Vindrola O, Baker C, Smith R. Pentylenetetrazol kindling produces a long lasting elevation of IR-Met-enkephalin but not IR-Leu-enkephalin in rat brain. Brain Res 1984;297:121-125 16. Vindrola O, Ase A, Finkielman S, Nahmod V. Differential release of enkephalin and enkephalin containing peptides from perfused cat adrenal glands. J Neurochem 1987; 50:424-430 Functional Neurology 2002; 17(2): 77-81 17. Eisenhofer G, Goldstein DS, Stull R et al. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenyl-alanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem 1986;32: 2030-2033 18. Tarchanoff J. Uber die galvanischen Erscheinungen in der Haut des Menschen bei Reigzungen der Sinnesorgane und bei verschiedenen Formen der pesychischen Tatigkeit. Pfleugers Arch 1890; 46:46-55 19. Shahani BT, Halperin JJ, Boulu P, Cohen J. Sympathetic skin response: a method of assessing unmyelinated axon dysfunction in peripheral neuropathies. J Neurol Neurosurg Psychiatry 1984; 47:536-542 10. Konishi S, Tsunoo A, Otsuka M. Enkephalin as a transmitter for presynaptic inhibition in sympathetic ganglia. 1981;294:80-82 11. Thoren P, Floras JS, Seals DR. Endorphin and excercise: physiological mechanisms and clinical implications. Med Sci Sports Exerc 1990; 22:417-428 12. Koltzenburg M. Afferent mechanisms mediating pain and hyperalgesias in neuralgia. In: Janig W, Stanton-Hicks M eds Reflex Sympathetic Dystrophy: A Reappraisal. Progress in Pain Research and Management, Vol 6. Seattle; IASP Press 1996:123-150 13. Drummond P, Finch P, Smythe G. Reflex sympathetic dystrophy: the significance of differing plasma concentrations in affected and unaffected limbs. Brain 1991;114:2025-2036 14. Baron R, Blumberg H, Janig W. Clinical characteristics of patients with complex regional pain syndrome in Germany with special emphasis on vasomotor function. In: Janig W, Stanton-Hicks M eds Reflex Sympathetic Dystrophy: A Reappraisal. Progress in Pain Research and Management, vol 6. Seattle; IASP Press 1996:25-48 15. Oyen WJG, Arntz IE, Claessens RAMJ, Van der Meer JWM, Corstens FHM, Goris RJA. Reflex sympathetic dystrophy of the hand: an excessive inflammatory response ? Pain 1993,55:151-157 16. Figuerola ML, Levin G, Leston J, Barontini M. Proenkephalin A and SNS activity in cluster headache under verapamil or prednisone treatments. Headache 1994,34:257-260 81