the long-term organ culture of tissues from adult amphiuma, the
advertisement

J. Cell Sci. I I , 799-813 (1972)
Printed in Great Britain
799
THE LONG-TERM ORGAN CULTURE OF
TISSUES FROM ADULT AMPHIUMA,
THE CONGO EEL
MARJORIE A. MONNICKENDAM AND M. BALLS
School of Biological Sciences, University of East Anglia, Norwich NOR 88C, England
SUMMARY
Fragments of pancreas, liver, spleen, kidney and lung from Amphiuma means, the Congo eel,
were maintained in organ culture for up to 35 days in a modified Leibovitz L15 medium. These
Amphiuma tissues retained their typical histological features throughout the culture period,
surviving better and for longer than tissues from other amphibian species. Liver and spleen
survived better in hypotonic or isotonic media (125-230 mosmol/kg) than in hypertonic media
(255-305 mosmol/kg). The mitotic incidences of these tissues in vivo were very low, and while
there were no significant increases in liver, spleen and lung fragments in vitro, there were large
increases in kidney and pancreas fragments. Amylase activity was found in pancreas fragments
and in medium from pancreas cultures after 28 days in vitro. Amphiuma organ cultures may
be useful in the in vitro study of the control of cell proliferation and cell function while normal
cell interactions are maintained.
INTRODUCTION
The control of cell differentiation, proliferation and function have been studied in
organ cultures where cells retain their 3-dimensional relationships with one another.
Cell-cell interactions are essential in morphogenesis; Tarin (1972) has discussed their
importance in morphostasis and their potential significance in carcinogenesis. Organ
cultures have been used to study mammalian morphogenesis; adult mammalian lung,
prostate, skin and intestine survive well, while liver, kidney, pancreas and spleen do
not.
In earlier experiments, we found that adult amphibian tissues from several species
survived well in long-term organ culture. The effects of culture on mitotic incidence
have been studied in the following: immature Xenopus epidermis, lung, spleen, liver
and kidney (Simnett & Balls, 1969); adult Xenopus pancreas and spleen, Rana spleen
and Triturus spleen (Balls, Simnett & Arthur, 1969); and Triturus pancreas, liver and
intestine (Monnickendam, Millar & Balls, 1970).
In the experiments to be described here, we used tissues from Amphiuma means.
The cells are large, and identifying mitoses or labelled cells in autoradiographs is
easier. Sufficient tissue can be obtained from one animal to set up many cultures,
since adult Amphiuma are large. We found that Amphiuma tissues survived well for
at least 35 days in vitro. We have looked at changes in the mitotic incidences of pancreas, liver, spleen, kidney and lung fragments, the effects of different media on mitotic
incidences and the retention of amylase activity by pancreas fragments.
51
CHLII
8oo
M. A. Monnickendam and M. Balls
MATERIALS AND METHODS
Animals
Adult Amphiuma were obtained from Carolina Biological Supply Company, Burlington,
North Carolina, U.S.A. They were kept in water at 18-23 °C an<^ fed on chopped beef liver and
heart.
Culture technique
Animals were anaesthetized in 1:100 MS222 (Sandoz) in water, the skin was then swabbed
with absolute ethanol. The pancreas, liver, spleen, lungs and kidneys were removed and cut
up with fine scissors into 2-3 mm cubes. The tissues were cultured separately in flasks containing 10 ml of medium, in free gaseous exchange with the atmosphere. Theflaskswere shaken
in a linear, simple harmonic shaker (Baird & Tatlock 'Unitemp' water bath with shaking
attachment) at a frequency of 2 cycles/s at 25 °C. The medium used contained: 50 % Leibovitz
Lis medium without NaHCO3 (Flow Laboratories, Irvine, Scotland), 8 % foetal bovine serum
(Flow Laboratories), 4 % tryptose phosphate broth, 38 % double-distilled deionized water,
100 i.u./ml Benzylpenicillin (Glaxo, Greenford, Middlesex), 100/ig/ml Streptomycin sulphate
(Glaxo), and 2 /'g/ml Fungizone (Squibb, New York).
The Li5, water and serum concentrations were varied in some experiments, but the other
constituents were kept constant. The medium was changed once a week. Colcemid (CIBA,
Basel) at a final concentration of 4/tg/ml, and [sH]thymidine (specific activity 5 Ci/mM; The
Radiochemical Centre, Amersham, Bucks.) at a final concentration of 5 /iCi/ml, were added to
cultures 8 h before the fragments were fixed. In each experiment, cultures were treated and
later fixed at the same times on appropriate days to minimize any variation due to diurnal
rhythms.
Histology and autoradiography
Tissues werefixedin Carnoy's fluid, embedded in paraffin wax, sectioned at 5 /Jm and stained
in Mayer's acid haemalum and eosin. Radioactive tissues were sectioned, dewaxed, dipped in
5 % trichloroacetic acid, and then coated with Ilford K2 nuclear research emulsion diluted
1:3 with distilled water. Slides were exposed for 2-4 weeks at 4 °C in light-tight boxes, developed
in Qualitol film developer (May & Baker) and stained in Giemsa.
Counting procedures
The mitotic and labelling incidences are defined as the number of mitoses or labelled cells
in a total of io 5 cells. The methods described by Simnett & Heppleston (1966) and Simnett &
Morley (1967) were used. In pancreas, only acinar cells were scored; in lung, only epithelium
was scored; in liver, spleen and kidney, all cells were scored, except for large blood vessels and
connective tissue masses. In general, 4 non-adjacent sections from each fragment were counted.
Results are given as arithmetic means with their standard errors. Where very few mitoses were
scored, the net mitotic incidence for the group is given, where:
net mitotic incidence =
total mitoses in the group x i o '
;
—: ;
.
total cells in the group
To compare mean values in different groups, the Student t-test was used, with a modification
for comparing means of 2 small samples (Bailey, 1959).
Assay of amylase activity
Amylase activity was assayed using a starch film method. Films were made by dipping clean
slides into a 4 % suspension of starch (Hydrolysed Starch, Connaught Laboratories, Toronto,
Canada) which had been heated to 100 CC for 15 min. The slides were then stood on end to
allow excess starch to drain away, and when dry were fixed overnight in methanol, acetic acid
Amphiuma tissues in organ culture
801
and distilled water (5:1:5 - Tremblay, 1963), then rinsed several times in distilled water and
allowed to dry. Qualitative tests for amylase activity were carried out by placing small pieces of
tissue on top of the starch with another slide on top of the pieces to prevent them from drying
out and then sticking to the film. Tissue fragments were left on the film for 30 min, then washed
off. Medium was tested by placing drops on the film, and washing off after 30 min. Treated
slides were stained in Gram's iodine solution, and amylase activity could be detected as clear
areas in the film due to enzymic hydrolysis of the starch.
Medium and Amphiuma serum osmolaUty
Osmolalities were measured by the freezing-point depression method using an Advanced
osmometer (Advanced Instruments, Newton Highlands, Mass.). Blood collected from 2 of the
Amphiuma used was allowed to clot, centrifuged and the osmolality of the serum measured.
The osmolalities of media containing 8% serum were, in mosmol/kg: 15% L15, 90; 30%
Li5. I25;4S% L15, 190; 60% L15, 230; 75% L15, 255 and 90% L15, 305.
RESULTS
Pancreas
The pancreatic exocrine tissue in Amphiuma is arranged in numerous acini, which
are not particularly well defined (Fig. 2). The mitotic incidence of acinar cells in vivo
is very low (less than 10). The small amount of endocrine tissue is not concentrated
into large masses, but is scattered throughout the acinar tissues in clusters of 2-10 cells.
The Amphiuma pancreas is particularly closely attached to the gut, so great care
was taken to avoid puncturing the gut and contaminating the tissue. Pancreas fragments survived remarkably well for long periods in vitro, retaining their normal
structure. In 50% L15 medium, there was a large increase in mitotic and labelling
incidences during 28 days in culture (Figs. 3, 4). Fragments were fixed on days 7, 14,
21 and 28, and the results are given in Fig. 1. No mitoses were found on day 7, and
the net mitotic incidence on day 14 was 10. On day 21, there was a 15-fold increase
in mitotic incidence, and a further significant increase on day 28 (P < o-ooi). The
mean labelling incidence was very low on day 7, but increased significantly on day 14
(0-02 < P < 0-05), with a further significant increase to a maximum on day 21
(0-02 < P < 0-05). There was no significant difference between labelling incidences
on days 21 and 28.
Amylase assays showed that amylase activity was present in pancreas fragments
assayed on days 17 and 28, and in the medium from day 28 cultures, which had been
in contact with the fragments from days 21 to 28. No amylase activity was found in
day 17 and 28 liver fragments or day 28 lung, kidney or spleen fragments, or in the
medium from any of these cultures (Fig. 5).
Liver
Amphiuma liver is a very heavily pigmented, cylindrical organ about 20 cm long
and 1-5 cm in diameter. It consists of polyhedral hepatocytes, numerous pigment
cells, Kupffer cells of the reticuloendothelial system, and subcapsular haematopoietic
tissue (Fig. 6). The mitotic incidence of the liver in vivo is very low (10), and most
mitoses occur in the subcapsular tissue.
51-2
M. A. Monnickendam and M. Balls
802
-, 10000
9000
8000
700
7000
600
6000
500
5000 <x>
0)
u
I 400
4000
c
u
5 300
3000
f
2000
200
1000
100
14
28
21
Time, days
Fig. i. Mitotic (101) and labelling ( • ) incidences of Amphiuma pancreas in vitro.
Total cells scored, 29 x io 6 . Vertical bars show means ±S.E.M.
Table 1. Mitotic incidence of Amphiuma liver: effects 0/L15 concentration
Day 7
t
Day 12
Li5, %
n
Mi
n
Mi
S*
25 ± 5
65 ± 20
6
8
9
i5± 5
io± 5
70 ±15
35 ±10
40 ± 10
30
9
45
9
60
7
75
9
30 ± 1 0
8
90
8
60 ± 20
8
Day 16
n
9
IO
Day 23
Mi
n
Mi
35 ± 10
25 ± 15
35 ± 1 5
9
9
7
9
9
7o±is
8
6 40 ± 10
80 ±30
45 ±25
45 ±20
9 45 ±15
25 ± 5
Total cells scored, 1 5 X io 5 ; n, number of fragments in the group; Mi, mitotic incidence
(mean ±S.E.M.); •, net mitotic incidence.
In vitro, Amphiuma liver fragments were well preserved for long periods in a wide
range of media, and, unlike Xenopus and Triturus liver fragments, did not develop
central necrosis. However, there was no significant increase in mitotic incidence in
vitro. Liver fragments were cultured in 30, 45, 60, 75 or 90% L15 medium with 8 %
serum and fixed on days 7, 12, 16 and 23. The fragments cultured in 30, 45 and 60%
AmpHuma tissues in organ culture
803
Table 2. Mitotic incidence of Amphiuma spleen: effects 0/L15 concentration
Day 7
Day 23
Day 12
A
A
Li5, %
n
Mi
n
30
45
60
75
90
6
6
io± 5
30 ± 5
50 ±15
50 ±15
35±i5
6
6
6
6
5
Total
5
6
6
cells scored, 23 x
6
IO .
Mi
70
45
65
65
25
±20
±15
±20
±25
±15
n
Mi
5
6
5
5
6
20 ± 10
35 ±10
30 ± 10
io± 5
2*
Symbols as in Table 1.
Li5 medium appeared perfectly normal, even after 23 days in vitro (Fig. 7), whereas
fragments cultured in 75 and 90% L15 medium showed marked deterioration after
16 days in vitro. There were no marked differences in mitotic activities in any of the
media (Table 1), although on day 7 higher mitotic incidences were found in fragments
from media with higher L15 concentrations, and on day 23 the higher mitotic incidences were in fragments from media with lower L15 concentrations. However, even
the highest mitotic incidence (80 + 30, day 23, 45 % L15) was very low compared with
those in liver fragments from other amphibian species (Monnickendam, unpublished
results). When liver fragments were cultured in 15, 30 or 45 % L15 medium with 8 %
or o-8% serum and fixed on days 14 and 21, it was evident that 15% L15 medium
was inadequate for maintenance of the tissue. No mitoses were found, the cells tended
to round off and became detached from one another, and pigment cells lysed, releasing
pigment granules throughout the fragments. In 30 and 45 % L15 the mitotic incidences
in media with 8% serum were higher than those in media with o-8% serum. On day
14, the highest mitotic incidence was found in fragments from 45 % L15, 8% serum
medium (net mitotic incidence = 20), and on day 21 the highest mitotic incidence
was found in fragments cultured in 30% L15, 8% serum medium (net mitotic
incidence = 70). Thus, although Amphiuma liver did not show a marked increase in
mitotic activity in vitro, the fragments survived and cells proliferated in a variety of
media with a wide range of osmolalities.
Spleen
The Amphiuma spleen, a cylindrical organ about 15 cm long and 0-5 cm in diameter,
is the major site of production of erythrocytes and thrombocytes in these animals.
In vivo, the mitotic incidence is very low (less than 10) (Fig. 8).
Spleen fragments were well preserved after long periods in organ culture in a
variety of media, though both mitotic and labelling incidences were low. When fragments were cultured in medium containing 70% L15 and 8% serum, and fixed on
days 11, 14 and 21, the net mitotic incidences were 20, 15 and 10 respectively, and the
mean labelling incidences were 250 ± 35, 75 + 30 and 50 + 20. There was a significant
decrease in the mean labelling incidence between days 11 and 21 (P < o-ooi). When
spleen fragments were cultured in media with 30, 45, 60, 75 or 90% L15 and 8%
804
M. A. Monnkkendam and M. Balls
Table 3. Mitotic incidence of Amphiuma kidney: effects 0/L15
and serum concentration
Medium
Day 21
Day 14
A
Li5,%
15
30
45
Serum, %
n
Mi
n
Mi
8
6
3*
6
< at
o-8
5
< 3t
6
f|
8
5
US ±35
6
905 ± H5
[
o-8
5
< 3t
6
225± 65
f
8
5
6
170± 50
7
6
335± 45
(
1
t o-8
45 ±15
5
Total cells scored, 5-8 x io ; n, number of fragments in the group; Mi, mitotic incidence
(mean±S.E.M.); # , net mitotic incidence; f , no mitoses in 3 x io4 or 5 x io4 cells respectively.
serum for 23 days, there was little change in mitotic incidence (Table 2). On day 7,
there were no histological differences between fragments cultured in the different
media, but by day 23, as in the liver cultures described earlier, it was evident that there
had been a marked deterioration of tissue structure in fragments cultured in 75 and
90% L15 medium, whereas those cultured in 30, 45 and 60% L15 medium were
indistinguishable from normal (not cultured) tissue, even after 30 days (Fig. 9). It is
evident from Table 2 that, excluding fragments cultured in 90% L15 medium the
mitotic incidence was low on day 7, increased slightly on day 12, and decreased by day
23. This fluctuation was most marked in 30% L15 medium. In 90% L15 the mitotic
incidence had decreased by day 12, and it was very low on day 23. With the exception
of 90% Li5 medium, which did not give good long-term cultures, there appeared to
be very little difference between fragments cultured in any of the media.
Spleen fragments were also cultured in 30 or 45% L15 medium containing 8 or
o-8% serum, and pieces were fixed on days 14 and 21. There were no obvious histological differences between fragments cultured in the various media, nor were there
significant differences between the mean mitotic incidences in fragments cultured in
medium with 30% L15, 8% serum and 45% L15 with 8 or o-8% serum. The mitotic
incidences of fragments cultured in medium with 30% L15, 0-8% serum were very
low (net mitotic incidence on days 14 and 2 1 = 2 ) compared with fragments cultured
in medium containing 30% L15 and 8% serum (mean mitotic incidence on day 14 =
20 + 5, day 21 = 40 + 5). The conclusion is that although there were no histological
differences between fragments cultured in the 4 media, mitotic activity was much
lower in fragments cultured in medium containing 30% L15 and o-8% serum.
Amphiuma spleen cells survived and proliferated in a variety of media with a wide
range of osmolalities, but, as in liver fragments, there was no marked increase in
mitotic activity in vitro.
Amphiuma tissues in organ culture
805
Kidney
Amphiuma kidneys are about 5 cm long and 1 cm wide, and the adrenal glands are
found as patches of yellow-orange tissue on the ventral surfaces of the kidneys. The
mitotic incidence of kidney in vivo is very low. A section of Amphiuma kidney is shown
in Fig. 10.
In vitro there was a large increase in mitotic incidence (Fig. 11), and the extent of
this increase was partially dependent on the culture medium used. Kidney fragments
were cultured in media containing 15, 30 or 45% L15 and 8 or o-8% serum, and were
fixed on days 14 and 21. The results are given in Table 3. There was no increase in
mitotic incidence in fragments cultured in medium containing 15% L15 and 8 or
o-8% serum, but there were large increases in fragments cultured in the other media.
On day 14, the mitotic incidence was higher in media with 30 and 45 % L15 and 8%
serum than in media with 0-8% serum, and no mitoses were found in 30% Li5,
o-8 % serum medium. Although the highest mean mitotic incidence was found in 45 %
Li5, 8% serum, it was not significantly higher than i n 3 0 % L i 5 , 8% serum. On day
21, there was an increase in mitotic incidence, compared with day 14, in both groups
cultured in media with 30% L15 (o-ooi < P < o-oi for 8% serum), and the mean
mitotic incidence was significantly higher in 8% than in o-8% serum (o-ooi < P <
o-o 1). There was also a significant increase in mean mitotic incidence on day 21,
compared with day 14, in both the 45 % L15 media (0-02 < P < 0-05 for 8% serum,
o-ooi < P < o-oi for o-8% serum); the mitotic incidence was also higher in o-8%
serum than in 8% serum, but this difference was not significant. The highest mitotic
incidence on day 21 was found in fragments cultured in medium containing 30% L15
and 8 % serum. Thus Amphiuma kidney cells survived in various media with a wide
range of osmolalities, but higher rates of proliferation were found in fragments
cultured in media with higher concentrations of L15 and serum.
Larger fragments of Amphiuma kidney tended to develop central necrosis in culture,
as did Xenopus kidney fragments (Simnett & Balls, 1969). However, this necrosis
developed later than in Xenopus kidney. Although there were no areas of dead cells
on day 11, it was evident that the central cells were no longer normal. The cytoplasm
stained comparatively lightly and the nuclei stained unevenly. On day 14, there were
dead areas in the centres of larger pieces. The extent of necrosis was dependent on the
culture medium, fragments cultured in media with lower L15 and serum concentrations having far larger necrotic areas.
Lung
Amphiuma lungs are cylindrical organs about 20-25 cm long and 0-5 cm in diameter.
In cross-section (Fig. 12) it is evident that the lung has a particularly thick layer of
fibrous supporting tissue with a central tube lined with epithelium (which shows a
low cell proliferation rate in vivo), with a few side branches into the supporting tissue.
Lung cultures were very well maintained, with normal histology after 35 days in
vitro (Fig. 13). There was no increase in the proliferative activity of the epithelium
in any of the media used. Lung fragments were cultured in media containing 30 or
8o6
M. A. Monnickendam and M. Balls
45 % L15 and 8 or o-8 % serum, and fixed on days 14 and 21; there were no significant
increases in mitotic incidences compared with fragments fixed directly from the
animal. Lung fragments cultured in 70% L15 medium and fixed on days 4, 6, 8 and 11
also showed no significant increase in mitotic activity. The conclusion is that lung
epithelial cells can survive for long periods in various media with a wide range of
osmolalities, but there is no increase in mitotic activity in vitro.
DISCUSSION
In general, the Amphiuma tissues used in these experiments survived better and for
longer than tissues from the other amphibian species used in our earlier experiments.
Amphiuma tissues were able to survive and proliferate in media with a wide range of
osmolalities (125-305 mosmol/kg), although some media were more suitable than
others. Liver fragments survived particularly well in media containing 8 % serum and
30-60% L15 (125-230 mosmol/kg), less well in 75-90% L15 media (255-305
mosmol/kg), and not at all well in 15% L15 medium (90 mosmol/kg). Spleen fragments behaved in a similar manner, and kidney fragments survived better and
developed higher mitotic incidences in 30-45 % L15 media than in 15 % L15 medium.
Hypotonic or isotonic media were more suitable than hypertonic media since the
osmolalities of sera from 2 Amphiuma were 224 and 222 mosmol/kg. Amphiuma red
blood cells haemolysed rapidly in hypertonic solutions (above 300 mosmol/kg) but,
though they swelled slightly, retained their integrity in hypotonic solutions (Dr Gillian
Rich, personal communication). Stocum (1968), looking at the differentiation of the
Ambystoma maculatum limb regeneration blastema in vitro, found that many media
which supported the survival of blastema cells did not support differentiation and
organogenesis. In particular, hypotonic media (200—240 mosmol/kg) merely allowed
blastema cell survival, whereas differentiation of precartilage and striated muscle was
observed in isotonic media (260 mosmol/kg). In our experiments, we have been
looking at the maintenance of normal tissue morphology, rather than the differentiation
of tissues, and the optimal conditions for maintenance (morphostasis) may well be
different from those required for development (morphogenesis).
Amphiuma pancreas and kidney fragments showed an increase in cell proliferation
in vitro compared with in vivo levels, a phenomenon which has also been observed in
organ cultures from other amphibian species. Adult Xenopus pancreas had a very low
in vivo mitotic incidence (less than 10), but this increased to 1535 on day 7 in raft
cultures (Balls et al. 1969). The mitotic incidence of adult Triturus pancreas in vivo was
also very low (15), and in flask cultures a peak incidence of 710 was observed on day 7
(Monnickendam et al. 1970). The increase in Amphiuma pancreas cell proliferation
developed more slowly, and the highest incidence was found after 28 days in vitro.
However, even after this long period in culture, amylase activity was still found in the
pancreas fragments, as well as in the culture medium. Since the medium was changed
weekly, amylase had been released into it between days 21 and 28. Immature Xenopus
kidney had a low in vivo mitotic incidence, which increased in vitro to 570 by day 6
(Simnett & Balls, 1969). Amphiuma kidney also showed an increased mitotic incidence
Amphiuma tissues in organ culture
807
in vitro; this developed more slowly than in Xenopus, and the extent of the increase
was dependent on the culture medium used. Although similar increases in mitotic
activity were found in immature Xenopus liver in vitro (Simnett & Balls, 1969) and in
cultured Triturus liver (Monnickendam et al. 1970), no such increase was found in
Amphiuma liver in any of the media used. Similarly, the mitotic incidences of Amphiuma
spleen and lung fragments did not increase in vitro. This low mitotic incidence may
explain our failure to detect antibody formation in Amphiuma spleens in vitro, whereas
adult Xenopus spleen fragments, which have much higher mitotic incidences in vitro,
can be induced to synthesize specific antibodies (Auerbach & Ruben, 1970).
The Amphiuma organ culture system has great potential value for studying the
control of cell proliferation and cell function in adult tissues, and the effects of hormones, drugs, carcinogens and viruses. In this system, normal cell-cell interactions
are maintained, and preliminary results indicate that cells, at least in some tissues,
continue their normal functions. In further papers, we shall discuss the maintenance
of normal cell ultrastructure, the relationship between cell size and metabolic rate, the
retention of tissue-specific enzyme patterns, and gametogenesis in cultured Amphiuma
material.
We gratefully acknowledge the receipt of a research grant from the Medical Research Council
of the United Kingdom.
REFERENCES
AUERBACH, R. & RUBEN, L. N. (1970). Studies of antibody formation in Xenopus laevis. J.
Immun. 104, 1242-1246.
BAILEY, N. T. J. (1959). Statistical Methods in Biology. London: English Universities Press.
BALLS, M., SIMNETT, J. D. & ARTHUR, E. (1969). Organ cultures of normal and neoplastic
amphibian tissues. In Biology of Amphibian Tumors (ed. M. Mizell), pp. 385-398. New York:
Springer Verlag.
MONNICKENDAM, M. A., MILLAR, J. L. & BALLS, M. (1970). Cell proliferation in vivo and in
vitro in visceral organs of the adult newt, Triturus cristatus carnifex. J. Morph. 132, 453-460.
SIMNETT, J. D. & BALLS, M. (1969). Cell proliferation in Xenopus tissues: a comparison of
mitotic incidence in vivo and in organ culture. J. Morph. 127, 363-372.
SIMNETT, J. D. & HEPPLESTON, A. G. (1966). Cell renewal in the mouse lung: the influence of
sex, strain and age. Lab. Invest. 15, 1793-1801.
SIMNETT, J. D. & MORLEY, A. R. (1967). Factors controlling growth of the prostatic epithelium:
a comparison of mitotic activity in mice of different ages in vivo and in organ culture. Expl
Cell Res. 46, 29-36.
STOCUM, D. L. (1968). The urodele limb regeneration blastema: a self organizing system. I.
Differentiation in vitro. Devi Biol. 18, 441-456.
TARIN, D. (1972). Tissue interactions in morphogenesis, morphostasis and carcinogenesis.
J. theor. Biol. 34, 61-72.
TREMBLAY, G. (1963). The localization of amylase activity in tissue sections by a starch film
method. J. Histochem. Cytochem. n , 202-206.
{Received 7 April 1972)
808
M. A. Moimickendam and M. Balls
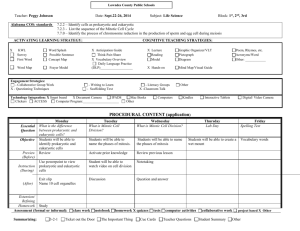
Fig. 2. Amphiuma pancreas (not cultured), showing acinar tissue, x 150.
Fig. 3. Day 28 pancreas, showing mitoses, x 380.
Fig. 4. Day 28 pancreas autoradiograph, showing nuclei labelled with [3H]thymidine.
X380.
Fig. 5. Starch film tests for amylase activity. A, fresh 50% L15, 8 % serum medium
(negative); B, medium from day 28 pancreas culture (positive); c, day 28 pancreas
fragments (positive).
Amphiuma tissues in organ culture
•*i
Ai^
8io
M. A. Monnickendam and M. Balls
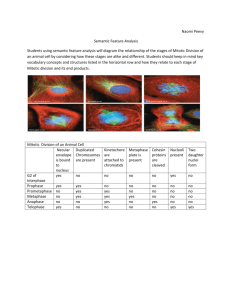
Fig. 6. Amphiuma liver (not cultured), showing hepatocytes (h), pigment cells (p)
and the subcapsular haematopoietic zone (s). x 150. Inset: hepatocytes. x 380.
Fig. 7. Day 23 liver, showing retention of normal histology, x 150. Inset: hepatocytes.
X380.
Fig. 8. Amphiuma spleen (not cultured), x 380.
Fig. 9. Day 30 spleen, x 380.
8n
Amphiuma tissues in organ culture
• * > •
*yyy
/
812
M. A. Monnickendam and M. Balls
Fig. 10. Amphiwna kidney (not cultured), showing a glomerulus (g) containing
erythrocytes, and tubules (t). x 240.
Fig. 11. Day 21 kidney, showing a glomerulus (g) and numerous mitoses, x 300.
Fig. 12. Ampliiuma lung (not cultured), showing epithelium and supporting tissue,
x 240.
Fig. 13. Day 35 lung, showing retention of normal histology, x 150. Inset: detail of
epithelium, x 380.
Amphiuma tissues in organculture
#
#'




![[#SWF-809] Add support for on bind and on validate](http://s3.studylib.net/store/data/007337359_1-f9f0d6750e6a494ec2c19e8544db36bc-300x300.png)