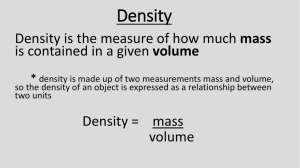
Name:
Date:
Unit 1 – Alchemy
Lessons 1 – 5 – Quiz Review
Directions: This study guide was designed to help you prepare for your quiz. Work
through each question carefully. Use your notes as a resource BEFORE you ask me
for assistance. Understand that you will need to APPLY what you’ve learned in
lessons 1-5 to your quiz questions.
Part I: Matter vs. Non-Matter: Determine if the things listed below are matter or not.
On the line provided, write Matter (M) or Not Matter (NM).
1. ____________________
Butter
2. ____________________
Cloud
3. ____________________
Dirt
4. ____________________
Anger
5. ____________________
Air
6. What is the definition of Matter?
Part II: Density: Read each question carefully. Write out your known values and
your unknown values, and then solve.
7. What is the definition of Density?
8. What is the formula for Density?
9. What would the triangle look like for the formula above?
10. How would you use the water displacement method to find the volume of a
sample? Be sure to include the proper names for the lab tools used.
11. What is the volume of an object that made the water level rise in a graduated
cylinder from 50 mL to 59 mL?
12. Imagine that the liquids on the right have these densities:
15g/cm3
3g/cm3
7g/cm3
10g/cm3
9g/cm3
12g/cm3
Label each layer with the correct density!
a) The liquids shown above are separated due to differences in
.
b) What is the density of the most dense substance in the beaker? ______________
c) What is the density of the least dense substance in the beaker? ____________
14. A sample of rubbing alcohol has a volume of 0.50 mL and has a mass of 0.41
grams. What is the density of this sample of alcohol? Show your work.
15. A 0.50 mL block of rock salt has a mass of 1.07 grams. What is the density of
this sample of rock salt? Show your work.
16. An 80.0 mL solution has a density of 5.80 g/mL. What is its mass? Show your
work.
17. What is the volume of 26.5 g of a solution whose density is 7.48 g/mL. Show
your work.
18. You have two objects of equal volume. Object A has a greater density than
object B. What can you say about the mass of object B?
Part III: Lab Safety: Circle the letter that best answers the question. Write an
explanation as to why you chose that answer.
19. You are heating a piece of glass and now want to pick it up. You should
a. use a rag or paper towel.
b. pick up the end that looks cooler.
c. use tongs.
d. pour cold water on it.
20. You have been injured in the laboratory (cut, burn, etc.). You should
a. visit the school nurse after class.
b. see a doctor after school.
c. tell your science teacher right away.
d. apply first aid yourself.
21. Personal eye glasses provide as much protection as
a. a face shield
b. safety goggles
c. splash - proof chemical goggles
d. none of the above.
22. In a laboratory, the following should not be worn.
a. Loose clothing
b. Dangling jewelry
c. Sandals
d. All of the above
Part IV: Laboratory Tools: Identify each tool below and explain the use of each.
23.
24.
26.
27.
25.
28.