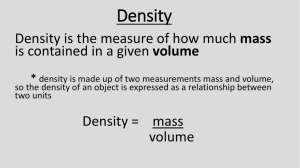Density Molecular View • The closer the molecules are in a given volume, the greater the objects density. Least Dense Density is a ____________ to _________ ratio Mass-200g Volume- 100cm3 Density- D = _____ g/cm3 Formula: Most Dense Mass– VolumeD= MassVolumeD=m/v = D = _____ g/cm3 D = _____ g/cm3 In Conclusion: • If an object is cut in half à _______________________________ • What type of property is density? __________________________ Density Calculations D= M= V= Example 1: A block of wood 3 cm on each side has a mass of 27 g. What is the density of the block? Example 2:What is the mass of an object if its density is 2 g/mL and its volume is 5 mL? Example 3: What is the volume of a liquid with a density of 2.0 g/mL and a mass of 8 g? 1 Example 4: What is density of a penny that weighs 2.49 grams and when placed in a graduated cylinder filled with water, the water rose from 10.0 mL to 10.5 mL. On Your Own: 1.) 5.0 mL of ethanol has a mass of 3.9 g, and 5.0 mL of benzene has a mass of 44 g. Which liquid is denser? 2.) What is the volume of your shoe- if the density is 0.85 g/mL and the mass is 60 grams. Density Column • When several immiscible liquids are combined, they form layers or a density column. • The liquids will order themselves according to their densities o smaller density à ___________ o larger density à __________ • Build your own Density Column! (directions on the screen) Color Density (g/mL) Object Density (g/mL) red 1.00 granite rock 2.75 green 0.75 plastic block 1.04 blue 1.10 Ivory soap 0.9 orange 1.25 wood block 0.65 2