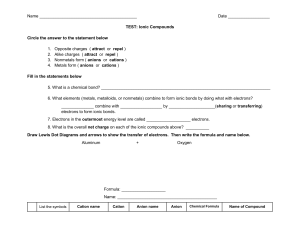
Grade-10 Science Name and Formula of Simple Ionic Compound -Handout for instruction Lesson objective: To learn about writing formula of simple ionic compounds Language objective: Read the background information given below, listen carefully to the example discussed by the teacher, observe on the board and write the examples discussed. ATL Focus: Communication Critical Thinking Background information: Metal loses electron and forms a positive ion (Positive ion is called cation). Non-metal gains electron(s) and forms negative ions called ‘anions’. Cations and anions are strongly attracted by electrostatic forces and forms ‘ionic compound’. All group 1 metals form cation with one positive (plus) (+) charge. Positive charge (+) is written on the top of the symbol of the metal as ‘superscript’, for example sodium ion is written as Na+ All group 2 metals forms cation with two positive charges (2+), For example – calcium ion is written as Ca2+. Transition metals forms ions with + or 2+ charge and it is given to you with the name by including roman number for example, iron (II) means Fe2+. Group 13 metals have 3+ charge. Non-metals form anions and have negative (minus) charge. Names of simple anions ends with the suffix ‘ide’; For example name of anion formed by chlorine is ‘chloride’ and it has 1- charge, formula of chloride ion is Cl-. All the halogens (group-17) have one negative charge. Group 16 anions will have 2- charge, example- anion of oxygen is called ‘oxide’ and its formula is O2- and Group 15 have 3- charge. For writing names – Always write the name of cations first and then anion. Example – Na+ and Cl- name is sodium chloride. Steps for writing formula: 1. Find the correct symbol from the periodic table. 2. Write the symbol of cation (positive ion). 3. Then write the formula of anion. 4. Balance the number of + and – charge by adding more cations/anions as required. 5. Write the number of additional ions as a ‘subscript’ after the formula. 6. Write the full formula (Do not include the charge of ions in the formula of compound). What to do next? – Try writing names and formula of simple ionic compounds in the worksheet provided.