Chapter 5 inorganic chem
advertisement

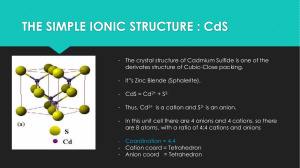
Chapter 5 – Ionic Solids (AxBy solids) http://www.quimica3d.com/en/access.php - animations of the structures that we talked about below Size of cations and anions; periodic trends in ionic radii Ionic crystal structures You will need to know the following structures: o cesium chloride o rock-salt (NaCl) o Zinc-blende (fcc/ccp ZnS) o fluorite (CaF2) for fcc/ccp: octahedral holes, tetrahedral holes (numbers and size) You need to be able to translate a description of a structure into a picture and calculate the fraction of octahedral and tetrahedral holes occupied as well as extract an empirical formula for a solid from the unit cell contents Determine the coordination numbers for the cation (to anions) and anion (to cations) Use radius ratio rules to make a prediction of what type of hole a cation (or the smaller ion) will occupy density calculations like for structures of metals. calculation of the size of various holes in the lattice. Intrinsic defects – Schottky and Frenkel Extrinsic defects -Fajan’s rules, polarizing ability of cations, polarization of anions. The bonding continuum concept and the bond triangle