Science I Chemistry: CM1000
advertisement
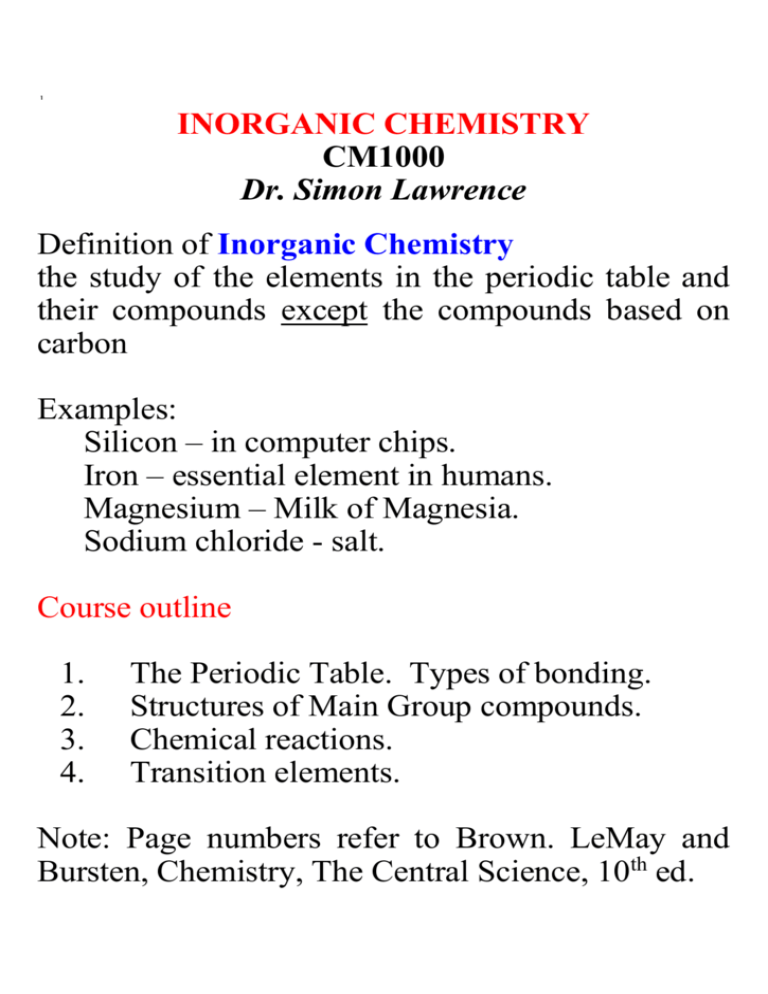
1 INORGANIC CHEMISTRY CM1000 Dr. Simon Lawrence Definition of Inorganic Chemistry the study of the elements in the periodic table and their compounds except the compounds based on carbon Examples: Silicon – in computer chips. Iron – essential element in humans. Magnesium – Milk of Magnesia. Sodium chloride - salt. Course outline 1. 2. 3. 4. The Periodic Table. Types of bonding. Structures of Main Group compounds. Chemical reactions. Transition elements. Note: Page numbers refer to Brown. LeMay and Bursten, Chemistry, The Central Science, 10th ed. 2 1.1. The Periodic Table - see Fig. 2.16, p. 50 Chap. 2, p. 49-51; Chap. 6, p. 246-249; Chap. 7 261-263 Groups with ns1 ....to ns2np5 are the main group elements (groups 1, 2, 12 to 17) Group 18 with ns2np6 are the noble gases or inert gases Groups with unfilled d shells i.e. ns2nd1 to 9 are the transition elements (groups 3 to 11) Look first at Main Group chemistry, later on at transition elements NB: Know the positions of the elements from H to Kr, i.e. atomic nos. 1 to 36. (Essential elements for life – see Fig. 2.24, p.59) 3 1. 2. Formation of Chemical Compounds. Chapter 8, p. 300-303 All chemical compounds are formed by the interactions of electrons in the valence shells of the elements involved. The valence shell is the outermost shell for Main Group elements, i.e. ns or ns np, e.g. N 2s 2p Valence shell electrons are the electrons in the outermost shell. When an element is involved in chemical bonding, it transfers or shares electrons so that its electron configuration is the same as the nearest Noble Gas (the Noble Gas Rule or Octet Rule) – see Prof. Brint’s course 4 1.2.1. Ionic Bonding. Chap. 2, p.55–59; Chap. 8, p. 303-304 One of the ways to combine elements is to transfer electrons to give ions. The compound formed is an ionic compound. The interaction of the ions is termed an ionic bond. Example: Na + Cl [Na+][Cl-] [3s1] [3s23p5] [3s0][3s23p6] NaCl is an ionic compound containing sodium cations and chloride anions. It is held together by the electrostatic attraction between the ions with opposite charges. Any system which has a positive charge is a cation e.g. Na+, Ca2+, [NH4]+. Any system which has a negative charge is an anion e.g. Cl-, O2-, [ClO4]-. 5 Metals will provide cations and non-metals will give anions. Cations and anions are present in living cells and in the environment. For example, seawater contains both Na+ and Cl- as does blood; Na+ and K+ play an important role in the transmission of nerve impulses. Cations Formed by elements on the left hand side of the periodic table, i.e. Groups 1,2, 12 and 13. Metals. All group 1 elements form M+ ions - ease of formation of M+ increases as the group is descended All group 2 elements form M2+ ions - ease of formation increases as the group is descended In group 13, Al and Ga form cations i.e. [Al]3+, [Ga]3+. 6 Anions Formed by elements on the right hand side of the periodic table, i.e. Groups (14), 15, 16 and 17. Non-metals. Group 17 forms X- ions for F, Cl, Br. Group 16 forms X2- for O, S, Se. Group 15 forms X3- for N, P, As. Group 14 forms X4- only for C (rare) Summary Ionic compounds may be formed between cations from Groups 1,2 or 13 and anions from Groups 15, 16 or 17, e.g. Na+Cl-, Ca2+S2-, Ga3+As3-. 7 Question Give the empirical formula for the ionic compounds formed from the following combination of elements. 1 2 3 4 5 Na and F Ca and S Ga and As K and O Al and O