Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division
advertisement

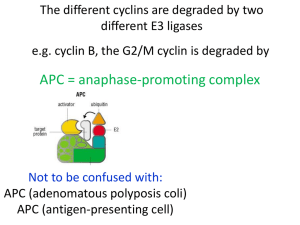
Chem*3560 Lecture 10: Cyclins, cyclin kinases and cell division The eukaryotic cell cycle Actively growing mammalian cells divide roughly every 24 hours, and follow a precise sequence of events know as the cell cycle. For simpler organisms like yeast, the cycle is similar, but is compressed to as little as 2 hours. The cell cycle was first described by cytologists, who described two distinct phases of the cycle, based on events that were directly observable through a microscope (Lehninger p.469). M phase (Mitosis) starts by dissolution of the nuclear envelope and condensation of chromosomes, which are initially in the form of replicate pairs. A series of dramatic and very visible events results in the division of the chromosome pairs , followed by orderly segregation carried out by the mitotic apparatus, so that the daughter nuclei each receive one copy of each chromosome. Finally two nuclei re-form around the chromosomes and the cell divides in two. During S phase (Synthesis) the DNA content of the cell doubles. The length of S phase depends to some extent on the size of the organism's genome Since no obvious change is visible in the microscope, the intervals from M to S and from S to the next M were labelled as Gap phases G1 and G2. At the biochemical level, the two gap phases are actually filled with activities such as expression of proteins, metabolism and synthesis of other structural components of the cell. Events in G2 are more specialized towards preparing for the coming mitosis. Cells that have ceased dividing exit the regular cell cycle, and enter a state called G0. G1 can be variable in duration, and is the phase that gets longer as cell growth slows down. A distinct point exists towards the end of G1 called Start or the restriction point. Once cells pass start, they proceed around the remaining stages of the cell cycle in a fairly orderly progression. However at various critical stages, there a checkpoints that only allow a cell to progress if they have satsfactorily completed a previous stage, so for example entry to G2 phase is stalled if DNA damage is detected, allowing time for repair. Specific protein kinases determine the timing of the cell cycle . Progress through the cell cycle is monitored and controlled by set of protein kinases whose activity rises and falls at different cycle phases. These operate through two distinct components, an activating protein called a cyclin, and the protein kinase catalytic subunit called a cyclin dependent kinase. Cyclins were first identified as proteins of unknown function whose concentration varied as a function of stage of the cell cycle, and go through a period of gradual build up followed by rapid protein destruction. The graph shows how cyclin B peaks at M phase, and is then destroyed. Genetic studies in fission yeast Schizosaccharomyces pombe provided the most important information from a set of mutations called cell division compromised or cdc. Mutations are a powerful way to study biological processes. Each mutant is observed so as to determine which biological function is lost, and then biochemical studies can identify which proteins are involved. In the case of cell division mutations, cell growth is arrested, so the mutations are derived as conditional or temperature sensitive (ts) mutants. The mutant functions perfectly normally allowing cell growth at what is called the permissive temperature , but becomes defective at the restrictive temperature , usually higher because thermal instability is the triggering factor. A yeast mutant first identified as cdc2 was found to be unable to progress through the cell cycle at the restrictive temperature. The cdc2 gene expressed a protein first identified as p34 (a protein recorded as 34 kDa on polyacrylamide gels. The p34 protein was found to function as a protein kinase that was only active in combination with a protein expressed by the cdc13 gene , which turned out to be a cyclin B homolog. Since the cdc2 protein is a protein kinase that requires a cyclin for activation, it was called Cyclin dependent kinase (CDK). Before long it was found that all eukaryotes express families of homologs of both CDK and cyclins. Different combinations of cyclins and CDKs control passage through different phases of the cell cycle. In mammals: Stage Targets Cyclin D-Cdk4 or 6 Progression through G1 phase Cell proliferation regulators Cyclin E-Cdk2 Entry to S phase DNA Replication proteins Cyclin A-Cdk2 Progression through S Cyclin A-Cdk1 Progression through G2 Cyclin B, CDK phosphatase Cyclin B-Cdk1 M-phase Chromosomal proteins Nuclear envelope proteins There are several other proteins that are cyclins or CDKs based on sequence similarities, but which have nothing to do with cell cycle regulation, e.g. Cyclin H and Cdk7 are part of the RNA polymerase II complex. Having found a regulatory mechanism that works, Nature uses it in many different ways. Cyclins are helix bundles, and share a common core of about 100 amino acids (blue/green) that make up the five helices that contact the CDK. Outside this region (yellow/red) there can be considerable variation, both in size and sequence. Cyclins can help determine which substrates are targets for the cyclin-CDK combination (in particular for yeast, where there is only one CDK for the whole cell cycle, and only the cyclins vary for different stages). CDKs have an N-terminal β-sheet that forms the ATP and substrate binding site, followed by the PSTAIRE helix which cyclins bind to. Hence PSTAIRE (these are the single letter codes of the amino acids in the helix) is a sequence signature to identify CDKs. This is followed by the Thr 160 loop, which blocks access to the substrate site in the absence of bound cyclin. When cyclin binds, the Thr 160 loop moves into contact with the cyclin, and this realigns Glu 51 correctly for its role in catalysis. Presence of cyclin activates CDK by a factor of 10,000. Finally, the C-terminal end of CDK forms another helix bundle (Lehninger p.470). CDK is itself regulated by phosphorylation. Each CDK is subject to a combination modes of regulation: by a specific pattern of phosphorylation within its own polypeptide; the required association with the activating cyclin; some CDKs can associate with factors called cyclin kinase inhibitors (CKI), which act by masking the kinase catalytic site with an autoinhibitory loop. CKI must be eliminated or inactivated for CDK to be available for catalysis. Different phosphorylation sites in CDK have different consequences for activity. Presence of phosphate on Tyr 15 occurs early, and keeps the CDK from being active prematurely. This phosphate is near the ATP substrate binding site, so the negative charges of Tyr-PO4 2– and ATP repel. Phosphorylation of Thr 160 helps shift the Thr loop out of the protein substrate binding site, and activates the CDK. This phosphorylation may be carried out by the cyclin-CDK combination controlling the preceding phase of the cycle. The M-phase cyclin B-Cdk1 combination must reach peak activity very rapidly, because M is the shortest of the four phases of the cell cycle. Cyclin B-Cdk1 phosphorylates and activates the phosphatase that removes its inhibitory Tyr-PO4 2-. This increases the activity of the CDK, which increases the activity of the phosphatase, a positive feedback loop. Positive feedback can be exploited to bring about very rapid transitions between two states (Lehninger, Fig 13-13, p.471.) Cyclin accumulates gradually but is destroyed rapidly Cyclin accumulates as its polypeptide is expressed (which may be controlled by the cyclin-CDK of a previous phase or by external growth factors). A common feature of Cyclin-CDKs is that they enable entry or progress through a stage of the cell cycle, but to complete passage through and terminate that phase, the cyclin must be destroyed. Targeted destruction of a protein is mediated by tagging it with a small protein called ubiquitin (ubique = Latin for everywhere). An enzyme called ubiquitin ligase links the C-terminal carboxylate of ubiquitin to a convenient lysine side chain of the doomed protein. Once a protein is tagged, it is picked up by a cellular complex called the proteasome , which uses ATP hydrolysis energy to unfold the polypeptide and feed it to a cluster of proteases. The essence of this mechanism is the ability of the ubiquitin ligase to identify which proteins are fated for destruction, so there are many different ubiquitin ligases in cells. Cyclin identifies itself as a target because it contains a sequence -Arg-Thr-Ala-Leu-Gly-Asp-Ile-Gly-Asn- called the cyclin destruction box. Each cyclin-CDK also activates an adaptor protein that is specific for linking that cyclin to its ubiquitin ligase, e.g. so that cyclin A can be destroyed while leaving cyclin B intact. Cyclin Kinase Inhibitors (CKI) keep cyclin kinase inactive if there's a problem CKI may be induced as a result of a cell not meeting the requirements of a cell cycle checkpoint. DNA damage triggers activity of a factor called p53 (protein of 53 kDa), which controls transcription of many genes, including CKI p21. CKI p21 binds to and inhibits Cyclin E-Cdk2, halting progress into S phase until the DNA damage is repaired. If the damage can't be repaired, p53 goes on to trigger a process called apoptosis, or programmed cell death.