Signalling Mechanisms of Cell Growth and Division
advertisement
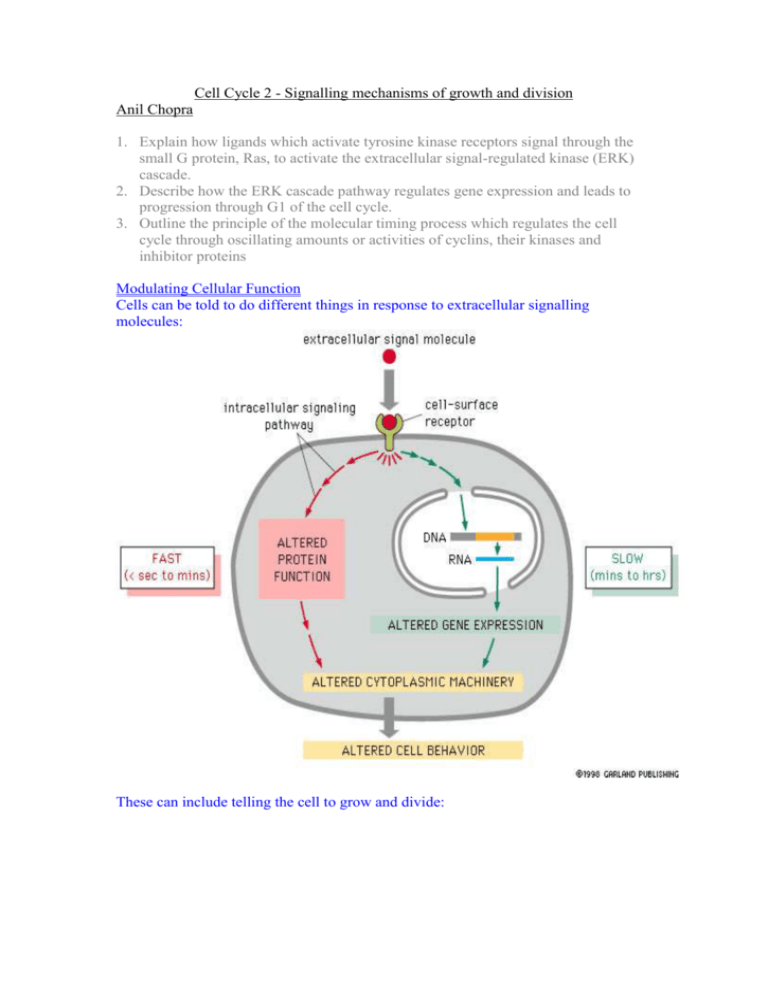
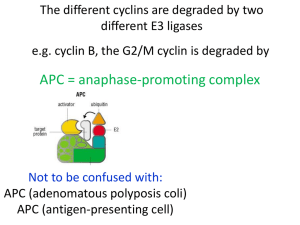
Cell Cycle 2 - Signalling mechanisms of growth and division Anil Chopra 1. Explain how ligands which activate tyrosine kinase receptors signal through the small G protein, Ras, to activate the extracellular signal-regulated kinase (ERK) cascade. 2. Describe how the ERK cascade pathway regulates gene expression and leads to progression through G1 of the cell cycle. 3. Outline the principle of the molecular timing process which regulates the cell cycle through oscillating amounts or activities of cyclins, their kinases and inhibitor proteins Modulating Cellular Function Cells can be told to do different things in response to extracellular signalling molecules: These can include telling the cell to grow and divide: Growth Process: 1. Hormones act on cell surface receptors resulting in the stimulation of pathways. 2. Active second messengers are produced resulting in amplification. (single receptor results in production of many other molecules) 3. Other factors are modulated and the produced molecules eventually result in many responses by the target cell. Key Components of Signalling Pathways 1. Protein Phosphorylation – transfer of terminal phosphate group from ATP to a hydroxyl group to form a phosphoester. a. Both kinase and phosphatase are regulated within the cell. Can turn proteins “on” or “off”. b. Effector proteins are also affected by phosphorylation. (works both ways). c. Kinase cascades exist: in which kinases activate kinases leading to signal amplification. 2. GTP Binding Proteins – molecular switches a. In its inactive state, it has a binding site that binds the nucleotide GDP b. When pathway activated GTP is made from GDP which changes the confirmation of the G-protein to its active form. Exchange Factors GTPase Activating Proteins (not kinases!) 3. Adapter Proteins a. Involved in binding other proteins together forming links between them b. Grb 2 - has different Src Homology domains SH3 Proline-rich regions (constitutive) regions SH2 Phosphorylated tyrosines (inducible) SH3 Growth Factor Stimulation Dividing cells are going around the cell cycle however most cells are in G0 when growth pathways are inactivated. No growth factors around. Cells in G0 enter the cell cycle e.g. in the process of wounding which results in the release of many growth factors. This causes cells to enter the cell cycle at G1. Mitogenic growth factor (i.e. growth signals from other cells) Receptor protein tyrosine kinase Small G protein (Ras) Kinase cascade Immediate early genes (c-jun, c-fos, c-myc transcription factors) – stimulate the expression of other genes (1) “myc” is a very important transcription factor and its level changes with differing levels of growth factor. Different growth factors include: - epidermal growth factor (skin) - platelet derived growth factor (2) When the receptors are in their inactive state they are “monomers”. The ligands are DIMERS which dimerise the receptors resulting in the transphosphorylation of the two monomers. - These phosphates are docking sites for signalling molecules. - Receptor has tyrosine kinase activity – Receptor Protein Tyrosine Kinase (RPTK) (3) The receptor becomes trans-phosphorylated on tyrosine (Tyr) residues and then adapter and signaling proteins are recruited. (e.g. Grb 2) (4) Receptor Protein Tyrosine Kinases (RPTKs) signal to Ras Grb 2 Exchange factor Sos (5) Ras - activates protein kinase cascade. - Known as MAPK cascade generically, however this particular one is the ERK cascade. - All this results in the changes in the target proteins. The amount of GTP-loaded Ras can be activated by oncogenes. This can result in mutations which eventually lead to an increased risk of tumour development. Specific Raf Generic MAPKKK (MKKK) MEK MAPKK (MKK) ERK MAPK How it Affects the Cell Cycle The cell cycle requires strict regulation: - complex checkpoints at different stages of the cycle Based on “Cyclin dependent kinases” (Cdks) in the proliferating cells. - Cyclins bind to these molecules. - These are expressed at certain points in the cell cycle. (by stimulation of genes) - Synthesised then degraded. Different cyclin Cdk complexes trigger different events in the cell cycle such as S phase (from the interaction of Cdk2 and Cyclin A) mitosis (Cdc2 + cyclin B) G1 progression (Cdk2 + Cyclin E). The complex formed by the binding of Cdk1 to mitotic cyclin is Mitosis Promoting Factor (MPF). In order for the MPF (Mitosis CdkCdk1 promoting factor) to become active, it needs two molecules CAK - Cdk Activating Kinase (CAK): a kinase that phosphorylates the activating kinase. - Wee1: an inhibitory kinase which removes the inhibitory phosphate. When these two molecules are present, the active MPF can form and result either in another Cdk being activated or for the next stage of the cell cycle to proceed. At different points in the cycle, different Cdks and cyclins are required to form complexes and trigger the next stage in the cell cycle. Cyclins are Requires activating phosphorylation sequentially synthesised so that AND removal of inactivating they trigger the expression of the phosphorylation genes necessary for the next phase of the cycle. P The complexes formed from Cdks and cyclins are used to phosphorylate proteins that are needed in the cell cycle e.g. - Nuclear lamins are proteins that breakdown the nuclear envelope but are not activated until Cdk1 binds to cyclin B. - Retinoblastoma proteins are used to inactivate transcription factors, however, when a complex formed by Cdk2 and cyclin E phosphorylates the retinoblastoma protein (Rb), then it becomes inactive and releases transcription factors: Rbs are TUMOUR SUPPRESSORS. E2 F Cdk4/6cyclinD Cdk2-cyclinE Inactive Transcription factor (e.g. cyclin E) Cdks are also regulated by Cdk inhibitors (CdkIs) e.g.: - INK 4 family - CIP/KIP family Active Transcription factor E2 F (e.g. cyclin E)