Reactions Class #4
advertisement
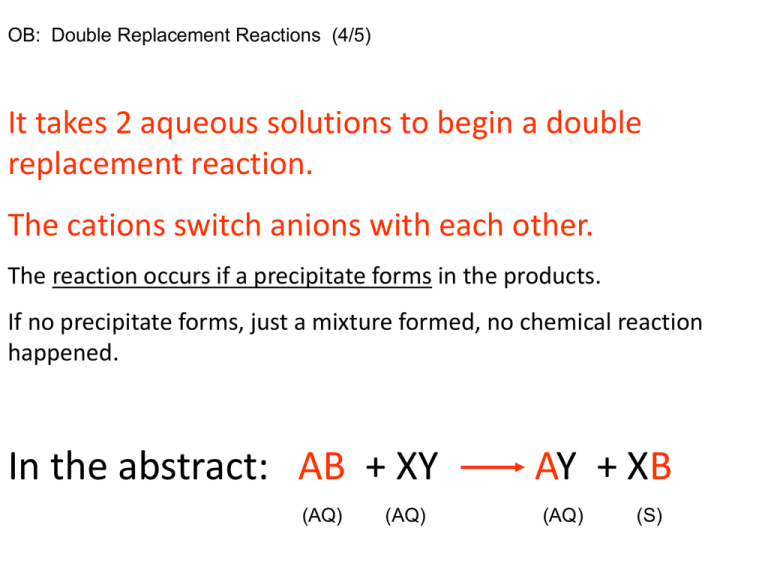
OB: Double Replacement Reactions (4/5) It takes 2 aqueous solutions to begin a double replacement reaction. The cations switch anions with each other. The reaction occurs if a precipitate forms in the products. If no precipitate forms, just a mixture formed, no chemical reaction happened. In the abstract: AB + XY (AQ) (AQ) AY + XB (AQ) (S) Must start with 2 AQ solutions, must end with 1 AQ solution + 1 SOLID PRECIPITATE How will you know if a product is soluble in water (dissolves), or insoluble (a precipitate)? Use… Take it out now… Example reaction: Copper (II) nitrate solution + ammonium carbonate solutions combine… First we must write the formulas correctly (watch ion charges) Cu+2 and NO3-1 then NH4+1 and CO3-2 Cations switch their Anions Cu(NO3)2(AQ) + (NH4)2CO3(AQ) NH4NO3 + CuCO3 Next, we check to see if our products are written properly according to ion charges Ammonium and nitrate are in 1:1 ratio OK, Cu+2 with CO3-2 also 1:1 OKAY Now we balance, then check table F for aqueous or solid Cu(NO3)2(AQ)+ (NH4)2CO3(AQ) 2NH4NO3 + CuCO3 The quick John Dalton, simple whole number ratio check is 1:1:2:1, okay. Let’s look at table F. What’s AQ, what’s S ? Cu(NO3)2(AQ) + (NH4)2CO3(AQ) 2 aqueous solutions to start 2NH4NO3(AQ) + CuCO3(S) 1 aqueous solution + 1 solid precipitate = DR Demo Diagram 4/5 Double Replacement Reactions 2 AQ solutions combine, if you get 1 (AQ) and 1 (S) precipitate to form then a double replacement reaction occurred. If 2 AQ solutions result in 2 different AQ solutions, nothing really happened. Abstract: FG(AQ) + XY(AQ) FY + XG [one AQ and one S!] Word equation: Sodium hydroxide and copper (II) sulfate solutions combine… Skeleton: Balanced: DIAGRAM for Double Replacement CuSO4(AQ) Blue solution Na2SO4(AQ) Clear solution NaOH(AQ) Clear solution Blue precipitate Cu(OH)2 (S) Every solution on the left side is soluble (dissolves in water). There are only a couple of exceptions. Halides are fluoride, chloride, bromide, and iodide solutions. Every solution on the right side is insoluble (solid precipitate in water) There are more than a few exceptions, but most of those you would already find on the right side (like group 1 ions, or ammonium, or nitrate, etc.) Always watch for the exceptions, let’s play now. Match the name to the formula, Table F each compound to decide AQ or S. • • • • • • • • • • Silver chloride Magnesium perchlorate Sodium hydroxide Calcium nitrate Strontium sulfate Barium acetate Aluminum chlorate Lead (II) bromide Lithium sulfide Ammonium chromate • • • • • • • • • • NaOH SrSO4 LiS (NH4)2CrO4 AgCl MgClO4 PbBr2 Ba(C2H3O2)2 CaNO3 AlClO3 Are these solutions soluble in water (AQ) or insoluble (S)? • • • • • • • • • • Silver chloride Magnesium perchlorate Sodium hydroxide Calcium nitrate Strontium sulfate Barium acetate Aluminum chlorate Lead (II) bromide Lithium sulfide Ammonium chromate • • • • • • • • • • AgCl(S) MgClO4(AQ) NaOH(AQ) CaNO3(AQ) SrSO4(S) Ba(C2H3O2)2(AQ) AlClO3(AQ) PbBr2(S) LiS(AQ) (NH4)2CrO4(AQ) Finish the word problems, do the formulas, balance the equations, complete the phase symbols for both products. Sodium chloride + lead (II) acetate solutions combine… Potassium phosphate + calcium chloride solutions combine… Finish the word problems, do the formulas, balance the equations, complete the phase symbols for both products. Sodium chloride + lead (II) acetate solutions combine… NaCl(AQ) + Pb(C2H3O2)2(AQ) 2NaC2H3O2(AQ) + PbCl2(S) Potassium phosphate + calcium chloride solutions combine… K3PO4(AQ) + 3CaCl2(AQ) 3KCl2(AQ) + Ca3(PO4)2(S) Finish the equations, balance the equations, complete the phase symbols for both products. BaCl2(AQ) + RbOH(AQ) NH4CrO4(AQ) + Ca(NO3)2(AQ) Finish the equations, balance the equations, complete the phase symbols for both products. BaCl2(AQ) + 2RbOH(AQ) NH4CrO4(AQ) + Ca(NO3)2(AQ) 2RbCl(AQ) + Ba(OH)2(S) 2NH4NO3(AQ) + CaCrO4(S) HW #4 Combustion Hand in Today, #3 DR, to be handed in on Tuesday. We will have a practice quiz NOW. Hurry. Watch Reactions Class #6 for Review, we will NOT do that slide show in class.