Biphenyl & Benzoic Acid Extraction: Lab Experiment
advertisement
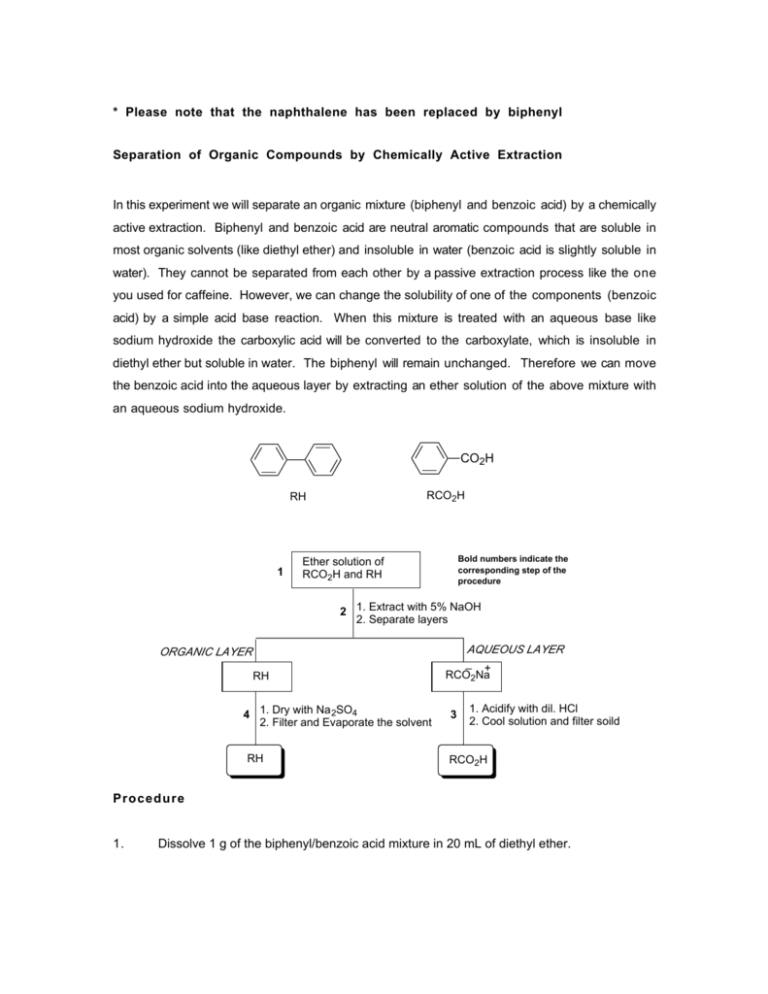
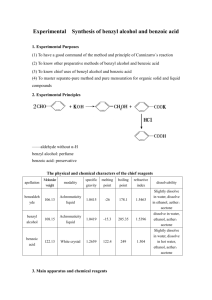
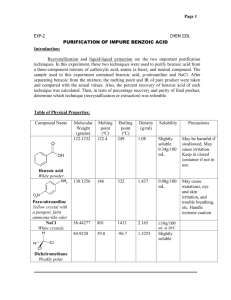
* Please note that the naphthalene has been replaced by biphenyl Separation of Organic Compounds by Chemically Active Extraction In this experiment we will separate an organic mixture (biphenyl and benzoic acid) by a chemically active extraction. Biphenyl and benzoic acid are neutral aromatic compounds that are soluble in most organic solvents (like diethyl ether) and insoluble in water (benzoic acid is slightly soluble in water). They cannot be separated from each other by a passive extraction process like the one you used for caffeine. However, we can change the solubility of one of the components (benzoic acid) by a simple acid base reaction. When this mixture is treated with an aqueous base like sodium hydroxide the carboxylic acid will be converted to the carboxylate, which is insoluble in diethyl ether but soluble in water. The biphenyl will remain unchanged. Therefore we can move the benzoic acid into the aqueous layer by extracting an ether solution of the above mixture with an aqueous sodium hydroxide. CO2H RCO2H RH 1 Bold numbers indicate the corresponding step of the procedure Ether solution of RCO2H and RH 2 1. Extract with 5% NaOH 2. Separate layers ORGANIC LAYER RH 4 1. Dry with Na 2SO4 2. Filter and Evaporate the solvent RH AQUEOUS LAYER _ + RCO2Na 3 1. Acidify with dil. HCl 2. Cool solution and filter soild RCO2H Procedure 1. Dissolve 1 g of the biphenyl/benzoic acid mixture in 20 mL of diethyl ether. 2. Pour this solution into a 125 mL separatory funnel and extract with 10 mL of 5% sodium hydroxide. Drain the aqueous layer carefully into a 125 mL erlenmeyer flask. Repeat this step and combine the aqueous layers. You have now separated the two compounds. The biphenyl is in the organic layer and the benzoic acid (in the form of the benzoate anion) is in the aqueous layer. 3. Neutralize the aqueous layer with dil. hydrochloric acid to regenerate benzoic acid from the benzoate anion. The hydrochloric acid should be added carefully using a plastic transfer pipette while swirling gently. The pH of the solution should be checked occasionally using litmus paper. Benzoic acid will precipitate when the aqueous layer becomes acidic. Cool the flask in an ice bath for 15 min. Filter the benzoic acid using a vacuum filtration. Allow the benzoic acid to dry on the buchner funnel for 20 min. and store it in your locker till the next lab. Weigh the benzoic acid and record its melting point. 4. Drain the organic layer into a 50 mL erlenmeyer flask and add a small amount (sufficient to form a 1/8" layer) of anhydrous sodium sulfate. This will remove traces of water from the organic layer. Allow the flask to stand for 10 min with occasional swirling. Decant the organic later carefully into a 50 mL beaker. Add a few boiling chips and evaporate the solvent on a hot plate (controller setting about 1.5). Remove the beaker when the solvent has evaporated. *Do not let the flask sit on the hot plate for too long after the solvent has evaporated. Biphenyl will crystallize upon cooling to RT. Weigh the biphenyl and record its melting point. Find the melting points of biphenyl and benzoic acid from the Aldrich catalog. Compare your melting points with the literature values.