Benzoic Acid Preparation Experiment
advertisement

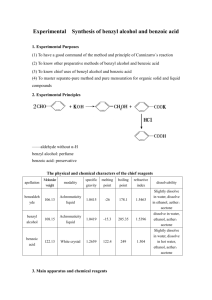
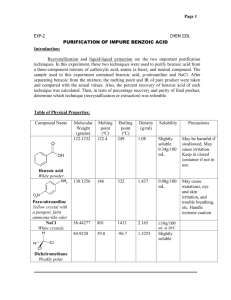
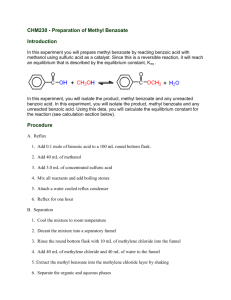
Experiment: The Preparation of Benzoic Acid A student carried out the preparation of benzoic acid by oxidizing 1cm3 of phenylmethanol (benzyl alcohol, C6H5CH2OH). The oxidation was carried out in alkaline conditions using sodium carbonate (Na2CO3) and an excess potassium manganate (VII) solution in a conical flask. After heating the solution for 20 minutes, it was cooled by running cool water over the outside of the flask. It was then acidified with concentrated hydrochloric acid and a brown precipitate formed. Sodium sulfite was then added until the precipitate disappeared and white crystals of benzoic acid became visible. The flask was placed in ice and the crystals were filtered off by vacuum filtration. The crystals were dried and weighed. It was found that 0.58g of benzoic acid was obtained. The balanced equation for the oxidation reaction is: 3C6H5CH2OH + 4KMnO4 3C6H5COOH + 4MnO2 + H2O + 4KOH (a) Why was an excess solution of potassium manganate (VII) used? (6) (b) Identify the brown precipitate formed in the reaction. (6) (c) Give two reasons why the solution was acidified with concentrated hydrochloric acid. (8) (d) How would you test the solution to find out if enough acid had been added? (3) (e) What reaction occurred when the sodium sulfite was added? (6) (f) Why was the flask placed in ice before filtration? (3) (g) How could the crystals have been dried? (3) (h) The phenylmethanol was the limiting factor in this reaction. Using the fact that the student used 1 cm3 of phenylmethanol (density = 1.04g/cm3 ) calculate: (i) the theoretical yield (12) (ii) the percentage yield if 0.58g of benzoic acid was obtained (3) Worked Answers (a) To ensure there was a sufficient amount of KMnO4 to oxidise all of the benzyl alcohol to benzoic acid. (b) A brown precipitate of MnO4 is formed as the Mn7+ ion is reduced to Mn4+ . (c) 1. To convert sodium benzoate to benzoic acid. 2. To neutralise any excess sodium carbonate. (d) Dip a glass rod into the conical flask and touch the end of the glass rod to a piece of damp blue litmus paper. The solution should be acidic (blue litmus paper turns red). (e) A reduction reaction (Mn4+ reduced to Mn2+). (f) To bring the benzoic acid crystals out of the solution. Benzoic acid has low solubility in cold water. (g) Place filter paper with crystals on clock glass and put them in an oven at a low temperature. (h) (i) mass = density x volume (1.04 g/cm3 ) (1 cm3 ) = 1.04g phenylmethanol. molarity = mass / molar mass. molar mass phenylmethanol = 108 g/mol. 1.04g / 108 g/mol = 0.00962 moles phenylmethanol. Look at the ratio in the balanced equation. There are three moles of phenylmethanol to every three moles of benzoic acid. So the theoretical yield of benzoic acid is 0.00962 moles. Let’s get that in grams. mass = molarity x molar mass molar mass benzoic acid = 122 g/mol. (0.00962) (122) = 1.17g benzoic acid (theoretical yield). (ii) percentage yield = ( actual yield / theoretical yield ) (100/1) (0.58g / 1.17g) (100 / 1) = 49.57% yield.