Exam #2 Key - De Anza College
advertisement
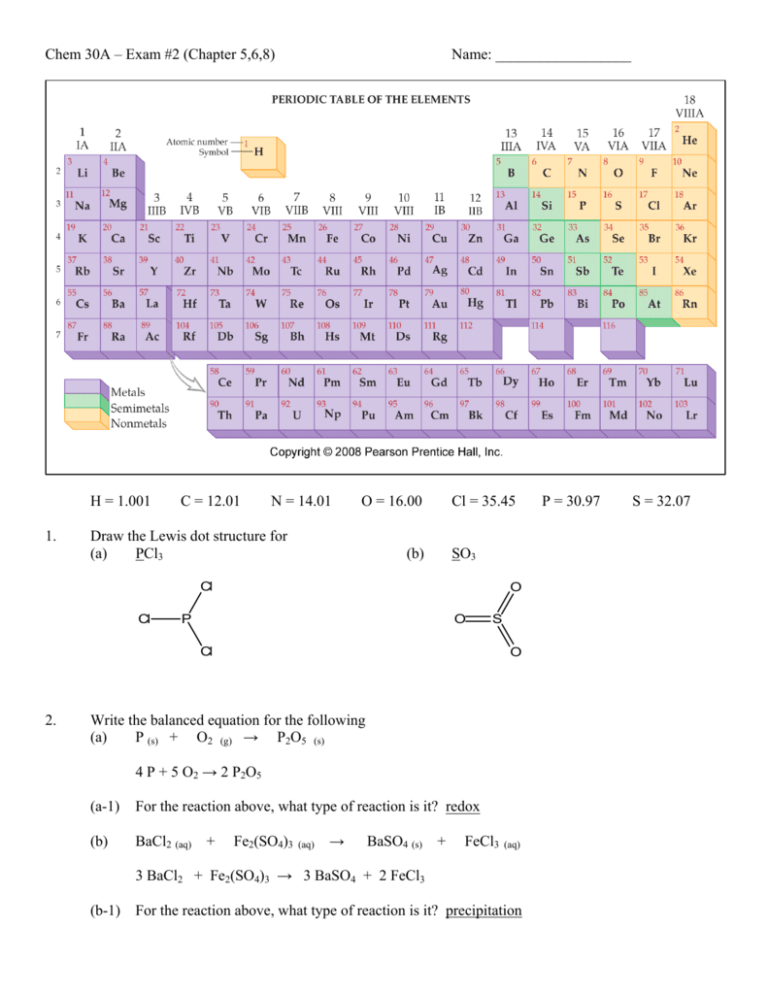
Chem 30A – Exam #2 (Chapter 5,6,8) Name: __________________ 04_05.JPG H = 1.001 1. C = 12.01 N = 14.01 O = 16.00 Draw the Lewis dot structure for (a) PCl3 Cl = 35.45 (b) SO3 Cl Cl O P O S Cl 2. O Write the balanced equation for the following (a) P (s) + O2 (g) → P2O5 (s) 4 P + 5 O2 → 2 P2O5 (a-1) For the reaction above, what type of reaction is it? redox (b) BaCl2 (aq) + Fe2(SO4)3 (aq) → BaSO4 (s) + FeCl3 (aq) 3 BaCl2 + Fe2(SO4)3 → 3 BaSO4 + 2 FeCl3 (b-1) For the reaction above, what type of reaction is it? precipitation P = 30.97 S = 32.07 3. Name the following compounds (a) P2O5 diphosphorous pentoxide (b) CCl4 carbon tetrachloride (c) N2O dinitrogen monoxide 4. What does STP stand for? 5. What are the values associated with STP? T = 0oC and P = 1 atm. 6. What is the volume of 3.0 mole of He at 760 torr and 30oC? standard temperature and pressure Problem recognition: PV = nRT moles given → likely ideal gas law problem. T = 30oC = 303 K P = 760 torr = 1 atm V = nRT / P = (3.0 mol)(0.0821 L atm / mol K)(303K) / 1 atm = 74.6 L = 75 L 7. What is the molar mass of N2O? MM of N2O = mass of all N + mass of all O = 2 (14.01) + 16.00 = 44.02 g / mol 8. What is the molar volume of N2O at STP? 22.4 L / mol 9. What value is associated Avogadro’s number? 6.02 x 1023 10. How many moles are present in 7.77 g of N2O? 7.77 g (1 mole / 44.02 g) = 0.177 mole 11. What is the mass of 777 molecules of N2O? 777 molecules ( 1 mole / 6.02 x 1023 molecules)(44.02 g / mol) = 5.68 x 10-20 g 12. At STP, what is the mass of 77 liters of N2O? 77 L ( 1 mole / 22.4 L )( 44.02 g / mol ) = 150 g 13. If 3.9 g of a gas has a volume of 22 L at STP, what is its molar mass? molar mass = mass / mole = 3.9 g / 0.9821 mole = 4.0 g / mol 22 L ( 1 mole / 22.4 L) = 0.9821 mole 14. If a tank of gas has a pressure of 600. torr at 25oC, assuming constant volume, what is the temperature of the gas, in Celsius, if the pressure increases to 1000. torr? Problem recognition: 2 sets of conditions, probably combine gas law problem constant volume = Gay-Lussac’s Law P1 / T1 = P2 / T2 (600. torr) / (298K) = (1000. torr) / T2 T2 = 497 K = 224oC 15. A tank containing a mixture of hydrogen, nitrogen, and helium gas has a pressure reading of 33 psi. If the pressures of hydrogen and nitrogen are 12 and 7 psi respectively, what is the pressure of the helium gas? Dalton’s law of partial pressure Ptotal = 33 psi = 12 psi + 7 psi + Phelium Phelium = 14 psi 16. What does the inside pressure become if an aerosol can with an initial pressure of 5.4 atm is heated in a fire from 20oC to 600oC? Problem recognition: 2 sets of conditions, probably combine gas law problem assuming constant volume = Gay-Lussac’s Law P1 / T1 = P2 / T2 (5.4 atm) / (293K) = P2 / (873K) P2 = 16 atm 17. Describe Boyle’s Law. The pressure and volume of a gas are inversely related under constant temperature. 18. Under what condition(s) do real gas behavior deviate from an ideal gas? pressure Given this balanced equation, N2 + 3 H2 → 2 NH3 low temperature and high 19. How many moles of N2 is needed to react fully with 7.2 g of H2? (7.2 g H2) ( 1 mole / 2.02 g H2) ( 1 N2 / 3 H2) = 1.2 mole N2 20. What is the theoretical yield of NH3 (in grams) that can be produced from 12.2 L of N2 at STP? (12.2 L N2) ( 1 mole / 22.4 L) ( 2 NH3 / 1 N2 ) ( 17.04 g / 1 mol NH3) = 18.6 g NH3 21. List the reactant(s) from the above balanced equation. N2 and H2 22. What is an aqueous solution? An aqeous solution is a solution containing one or more compounds dissolved in water. 23. Between boron (B), carbon (C), and nitrogen (N), which is the most least electronegative element? (circle your answer) -- electronegativity decreases going left on the periodic table. 24. Given the electronegativity of phosphorous and chlorine are 2.1 and 3.0 respectively, is the P-Cl bond polar or non-polar? (circle the answer) difference in electronegativity (3.0 – 2.1 = 0.9) greater than 0.5 25. Attribute the following properties to either ionic (I) or molecular (M) compounds. (a) low melting point molecular (b) many are water soluble ionic (c) can be gases, liquids, or solids molecular 26. Predict the solubility of the following compounds in water as either soluble (S) or insoluble (I). (a) CaCO3 insoluble soluble (according to text) (b) CaSO4 (c) Li2SO4 soluble (d) CaCl2 soluble (e) AgCl insoluble 27. List in order of increasing strength the following types of intermolecular forces. Dipole-dipole interaction, Hydrogen bonding, and London dispersion forces. L.D.F → D.D. → Hydrogen bonding 28. A weather balloon is filled with helium to a volume of 225 L at 18oC and 763 mmHg. The balloon ascends to an altitude where its volume rises to 1365 L and the temperature is -55oC. What is the pressure at this altitude? 2 sets of conditions, combine gas law P1 V1 / T1 = P2 V2 / T2 (763 mmHg) ( 225 L) / (291K) = P (1365 L) / 218 K P = 94.2 mmHg









