__________________
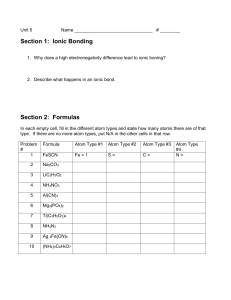
___________
__________
___________
___________
________
________
_____
____
____
_____
___
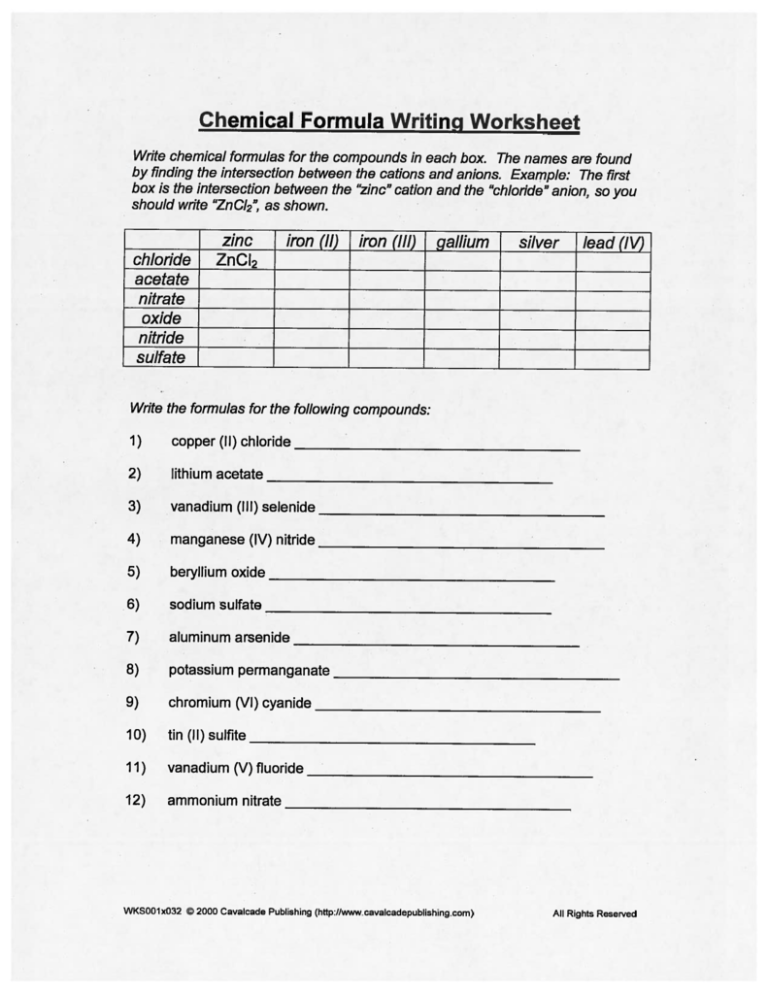
Chemical Formula Writing Worksheet
Write chemical formulas for the compounds in each box. The names are found
by finding the intersection between the cations and anions. Example: The first
box is the intersection between the “zinc” cation and the “chloride” anion, so you
should write “Zn Cl
”, as shown.
2
zinc
chloride
iron (II)
iron (III)
gallium
silver
lead (IV)
2
ZnCI
acetate
nitrate
oxide
nitride
sulfate
Write the formulas for the following compounds:
1)
copper (II) chloride
2)
lithium acetate
3)
vanadium (Ill) selenide
4)
manganese (IV) nitride
5)
beryllium oxide
6)
sodium sulfate
7)
aluminum arsenide
8)
potassium permanganate
9)
chromium (VI) cyanide
10)
tin (II) sulfite
11)
vanadium (V)fluoride
12)
ammonium nitrate
—
WKSOO1xO32 © 2000 Cavalcade Publishing (http:llwww.cavalcadepublishing.com)
All Rights Reserved
_________________________________
___________
___________
___________
____________________________
___________
___________
___________
_______
_____
__________________________________
___________
___________
_______________________
___________
___________
__________
________
___________
_____________________
____________________
__________________
________________
_______________
___________
___________
__
_______
___________
___________
_________________________________
__________________________
________________________
_______________________
___________
___________________
_______________
___________
___________
_________
________
________
SNC2DI
Name:____________
Naming and Writing Formulas of Ionic Compounds
1. Name each of the following compounds using the “ous-ic” method where applicable.
a) CuCl
2
e) MgBr
b) Fe
2
N
3
f) ZnO
c) PbQ
g)
AlP
d) HgF
2
h)
CaS
2. Name each of the following compounds using the roman numeral method where applicable.
2
C
_
3
____________________
a) 0
r
_________ e)
b)
Co5
5
9
H
2
2
P
3
f) Zn
c) Ag
0
2
2
g) PbCI
d) SnF
4
h) Bi205
3. Write the formula for each of the following compounds.
a)
chromous
e) ferric fluoride
oxide
b) plumbic sulfide_____________________
f)
stannic carbide
c) sodium bromide
g) silver
d)
cobaltous
h)
4.
Write
a)
b)
c)
d)
nickel
chloride
the
formula for each of
(III)
bromide
aluminum oxide
manganese
chromium
(IV) sulfide
(II) sulfide
the
iodide
nickelous
iodide
following compounds.
chloride
e) potassium
f)
iron
(III)
g) arsenic
h)
phosphide
(V)
antimony
oxide
(III)
fluoride
I
-
fO’ H I
‘A
‘
:
A1’•s: ‘i Sot *
Write chemical formulas for the compounds in each box. The names are found
by finding the intersection between the cations and anions. Example: The first
box is the intersection between the “zinc” cation and the “chloride” anion, so you
should write “Zn Cl
”, as shown.
2
zinc
iron (II)
chloride
2
Cl
acetate
nitrate
oxide
:;
iron (Ill)
.FI:
gallium
nitride
ZnO
2
N
3
Zn
FeC)
2
N
3
Fe
sulfate
4
ZnSO
4
FeSO
F
Fe-*
F
-,
lead (IV)
PFCl
3
GaOl
)
2
GC’HO
‘:
silver
,q
...
)
3
Ga(N0
AgNO
Ga
3
0
2
GaN
Ag
0
2
N
3
Ag
SO
2
Ag
4
Pb(NO)
PV
_
31
Pb
Pb(S0
2
)
4
Write the formulas for the following compounds:
1)
copper(ll)chloride CuC.
2)
lithium acetate LiC
O
3
H
2
3)
vanadium (Ill) selenide VSc
4)
manganese (IV) nitride Mn
4
N
3
5)
beryllium oxide BeO
6)
sodium sulfate Na
4
S
2
O
7)
aluminum arsenide AlAs
8)
potassium permanganate KMnO
4
9)
chromium (VI) cyanide Cr(CN)
6
10)
tin (II) sulfite SnSO
3
11)
vanadium (V) fluoride VF
5
12)
ammonium nitrate 4
NH
N
C)
WKSOO1x032 © 2000 Cavalcade Publishing (http://www.cavalcadepubIishing.com)
All Rights Reserved
____________f)
________
Naming and Writing Formulas of Ionic Compounds
1. Name each of the following compounds using the ‘ous-ic” method where applicable.
a) CuCI _cuprous chloride__________ e) MgBra magnesium bromide___________
b) Fe
2 _ferrous nitride
N
3
f) ZnO _zinc oxide____________________
c) PbO ._plumbous oxide_____________ g) AlP _aluminum phosphide
d) H
2 _mercuric fluoride___________ h) CaS _calcium sulfide________________
F
9
2. Name each of the following compounds using the Roman Numeral method where
applicable.
a) 2
Cr
_
3
0
chromium (III) oxide_______ e) Hg
5 _mercury (I) sulfide
2
b) CoS
—
cobalt (II) sulfide
2 _zinc phosphide
P
3
Zn
92 _silver oxide________________ g) PbCI
A
c) 0
2 _lead (II) chloride____________
d) SnF
4 _tin (IV) fluoride_____________ h) Bi
5 _bismuth (V) oxide___________
0
2
3. Write the formula for each of the following compounds.
a) chromous oxide
b) plumbic sulf ide
Cr0______________ e) ferric fluoride _FeF
_________________
3
PbS,
f) stannic carbide _SnC__________________
c) sodium bromide _NaBr
0
2
g) silver iodide _Ag
d) cobaltous chloride _CoCI,
h) nickelous iodide _NiI,
4. Write the formula for each of the following compounds.
a) nickel (III) bromide .NiBr
3
e) potassium chloride _KCI_______________
b) aluminum oxide _Al
_____________ f) iron (III) phosphide ......FeP___________
3
0
2
c) manganese (IV) sulfide MnS
2
g) arsenic (V) oxide _As
_____________
5
0
2
d) chromium (II) sulfide _CrS___________ h) antimony (III) fluoride _5bF
______
3