PHGN341: Thermal Physics PhET Virtual Experiment on the Ideal
advertisement

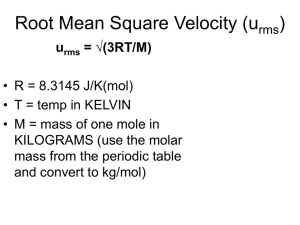
PHGN341: Thermal Physics PhET Virtual Experiment on the Ideal Gas Due: January 25, 2013 NAME: In this virtual experiment we will use an ideal gas simulator to study effusion (leaking). Go to “http://phet.colorado.edu/en/simulation/gas-properties” and launch the Gas Properties applet. Run the applet and get familiar with the various controls, parameters, and measurement tools. Launch the ruler and make the chamber about 8 nm long. With the constant parameter set to “None”, add 240 heavy molecules to the chamber and watch the pressure and temperature until they settles down. Add or subtract particles/heat to get as close as you can to the standard pressure and temperature values of 300 K and 1 atm. When you are reasonably close, fix the temperature by clicking on the “Temperature” button in the “Constant Parameter” box. This will be your “standard” starting condition. 1. Launch the species information tool to get an estimate of the average speed of the heavy molecule and use this to estimate the mass of the heavy molecule in amu’s, mH . From an estimate of the variance in the speed measurement, estimate the variance in your mass calculation and record your results below. (1 amu = 1.66 × 10−27 kg) mH = ± amu. 2. Launch the stop watch. Place the ruler near the lid (on top of the chamber) and move the lid approximately 1 nm and start the stop watch. You will see molecules start to leak out. Monitor the number of particles until it reaches half your starting number and record the effusion half-life for the heavy molecule, τH . Repeat this a couple of times to get an estimate of the variance and record your estimated values below. (Did you notice how heat is occasionally added to keep the temperature constant. Why is that?) τH = ± ps. 3. Starting from the “standard” condition for light molecules, repeat these measurements for the light molecules. mL = ± amu. ± τL = ps. 4. Based on the kinetic theory model for effusion (HW Problem 1.22), what would you expect for the ratio of the effusion half-lives. Compare that with the value you measured. τL τH = τL τH (theory expectation). 5. What other experimental test of the effusion model can you think of? 1 = (experiment).