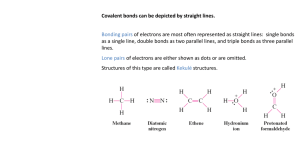
Lewis Structures & Resonance Homework
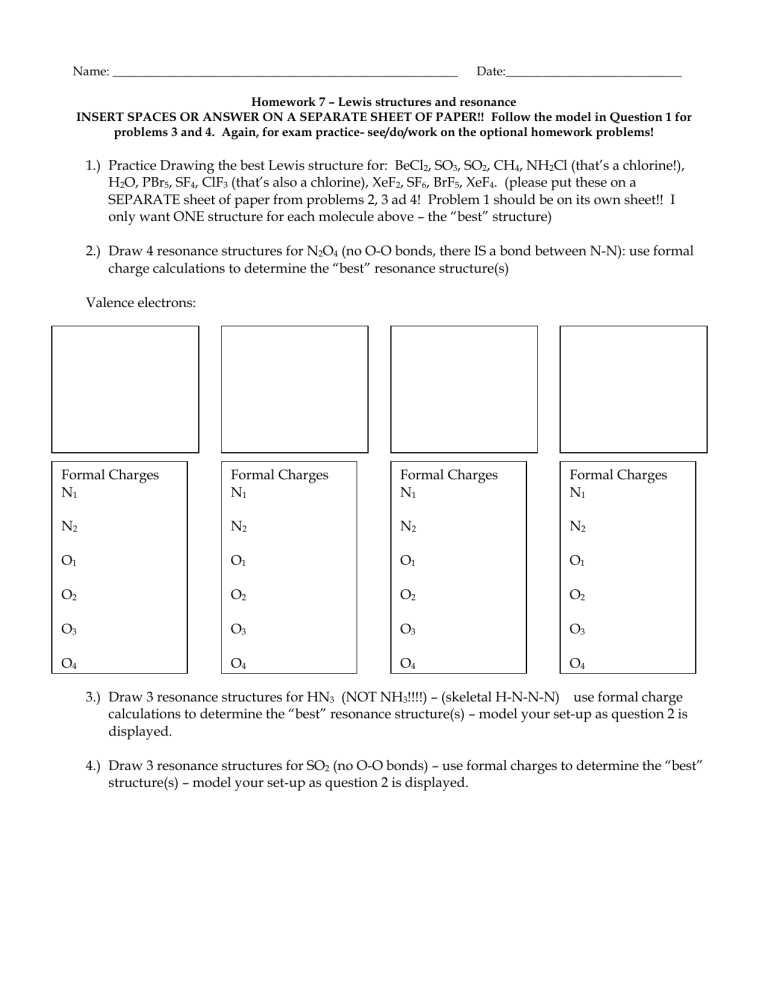
Name: _______________________________________________________ Date:____________________________
Homework 7 – Lewis structures and resonance
INSERT SPACES OR ANSWER ON A SEPARATE SHEET OF PAPER!! Follow the model in Question 1 for problems 3 and 4. Again, for exam practice- see/do/work on the optional homework problems!
1.) Practice Drawing the best Lewis structure for: BeCl
2
H
2
O, PBr
5
, SF
4
, ClF
3
(that’s also a chlorine), XeF
2
, SF
6
, SO
, BrF
3
5
, SO
2
, XeF
, CH
4
4
, NH
2
Cl (that’s a chlorine!),
. (please put these on a
SEPARATE sheet of paper from problems 2, 3 ad 4! Problem 1 should be on its own sheet!! I only want ONE structure for each molecule above – the “best” structure)
2.) Draw 4 resonance structures for N
2
O
4
(no O-O bonds, there IS a bond between N-N): use formal charge calculations to determine the “best” resonance structure(s)
Valence electrons:
Formal Charges
N
N
O
O
O
O
1
2
1
2
3
4
Formal Charges
N
1
Formal Charges
N
1
Formal Charges
N
1
N
2
O
1
N
2
O
1
N
2
O
1
O
2
O
3
O
2
O
3
O
2
O
3
O
4
O
4
O
4
3.) Draw 3 resonance structures for HN
3
(NOT NH
3
!!!!) – (skeletal H-N-N-N) use formal charge calculations to determine the “best” resonance structure(s) – model your set-up as question 2 is displayed.
4.) Draw 3 resonance structures for SO
2
(no O-O bonds) – use formal charges to determine the “best” structure(s) – model your set-up as question 2 is displayed.