Lewis Structures & Molecular Properties Homework
advertisement

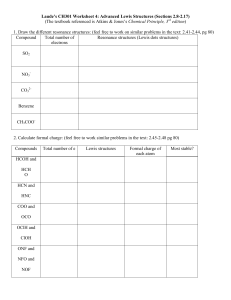
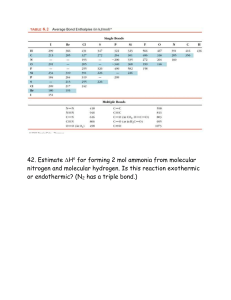
HW 5D 1. Write the Lewis structures for the following. Show all resonance structures where applicable. a. NO2e. O3 b. N2O4 f. SO2 c. OCNg. SO3 d. N3- 2. Benzene (C6H6) consists of a six-membered ring of carbon atoms with one hydrogen bonded to each carbon. Write Lewis structures for benzene, including resonance structures. 3. A toxic cloud covered Bhopal, India in December 1984 when water leaked into a tank of methyl isocyanate and the product escaped into the atmosphere. Methyl isocyanate is used in the production of many pesticides. Draw the Lewis structures for it, CH3NCO, including resonance forms. 4. Order the following species with respect to carbon-oxygen bond length, longest to shortest. Then list the order from the weakest to the strongest bond. CO, CO2, CO32-, CH3OH 5. Use the formal charge arguments to rationalize why BF3 would not follow the octet rule. 6. Use formal charge moments to explain why CO has a much smaller dipole moment than would be expected on the basis of electronegativity. 7. Oxidation of the cyanide ion produces the stable cyanate ion, OCN-. The fulminate ion, CNO-, on the other hand, is very unstable. Fulminate salts explode when struck; Hg(CNO)2 is used in blasting caps. Write the Lewis structure for both ions. Why is fulminate ion so unstable? 8. When molten sulfur reacts with chlorine gas, a vile-smelling orange liquid forms that has an empirical formula of SCl. The structure of the compound has a formal charge of zero on all elements in the compound. Draw the Lewis structure for the vile-smelling orange liquid. 9. Nitrous oxide has three possible Lewis structures. Draw the three resonance structures. Given the following bond lengths, rationalize the observations that N-N bond length in N2O is 112 pm and that the N-O bond length is 119 pm. Assign formal charges to the resonance structures for N2O. Can you eliminate any of the resonance structures on the basis of formal charge? Is this consistent with observation? N-N 167 pm N=N 120 pm 110 pm N=O 115 pm N-O 147 pm 10. A common trait of simple organic compounds is to have Lewis structures where all atoms have a formal charge of zero. Consider the following incomplete structure for an organic compound called methyl cyanoacrylate, the main ingredient in super glue. Draw a complete Lewis structure for the compound in which all atoms have a formal charge of zero. 11. Benzoic acid, C6H5CO2H, is a food preservative. Draw the Lewis structure, including all resonance structures in which all atoms have a formal charge of zero.