4 Polyatomic Ions And Transition Metals
advertisement

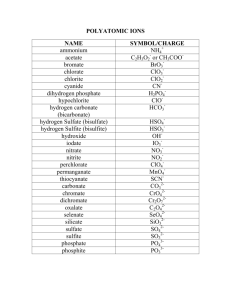
Pirate Chemistry Polyatomic Ions We saw in the previous section how monatomic ions (1 atom per ion) are named and put together. There are other types of ions, though, that are more complicated. These are called polyatomic ions– multiple atoms that share a combined charge. How and why these atoms group together to make one charge is not important right now. What is important is how they are named and how they go together. Most polyatomic ions are anions; ions with a negative charge. Below is a list of many of them: Negative Ions (anions) Acetate C2H3O2 Bicarbonate HCO3-1 Carbonate CO3-2 -1 Chlorate ClO3 Chlorite ClO2-1 Chromate CrO4-2 Cyanide CN-1 Cyanate CNO-1 Dichromate -1 -2 Cr2O7 Dihydrogen phosphate H2PO4-1 Hydrogen phosphate HPO4-2 Hydrogen sulfate HSO4-1 Hydroxide OH-1 Hypochlorite -1 ClO -1 Nitrate NO3 Nitrite NO2-1 Oxalate C2O4-2 Perchlorate ClO4-1 Permanganate MnO4-1 Peroxide O2-2 Phosphate PO4-3 Phosphite PO3 -3 Sulfate SO4-2 Sulfite SO3-2 Thiocyanate SCN-1 Thiosulfate S2O3-2 Positive Ions (cations) Ammonium NH4+1 Ammonium is the only polyatomic cation we will use. Notice how most of these ions end in either the “-ate” or “-ite” endings. In the previous section we only had the “-ide” ending. Remember that if you have a monatomic ion (straight from the periodic table and not from this list), it will always end with the “ide” suffix. Parentheses When we combine polyatomic ions, we will often have to use parentheses to correctly list the formula. We use parentheses when we have multiple polyatomic ions. Let’s look at an example: Calcium and Nitrate Ca+2 NO3-1 Nitrate is on the list opposite. Ca NO3 Criss-cross the charges Ca NO3 2 There is one calcium There are two nitrates Right now it looks like 32! Use parentheses Ca(NO3)2 Final formula One calcium Two nitrates Let’s practice this a bit on the next page. All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org, and it will be. Pirate Chemistry Example 1: K+1 Potassium Permanganate MnO4-1 KMnO4 Example 2: These charges cancel each other out. Final formula Magnesium Sulfate Mg+2 SO4-2 These charges cancel each other out. MgSO4 Final formula Example 3: Sodium phosphate Na+1 PO4-3 These charges DON’T cancel each other out. Na PO4 Criss-cross the charges Na3PO4 Final formula Notice that we don’t use parentheses We only use parentheses when we have multiple polyatomic ions Example 4: Aluminum dichromate Al+3 Cr2O7-2 These charges DON’T cancel each other out. Al Cr2O7 Criss-cross the charges Al2(Cr2O7)3 Final formula Notice that we don’t use parentheses for the aluminum. It’s not a polyatomic ion. Notice that we do use parentheses for the dichromate. We have three of them and it is a polyatomic ion. Example 5: Ammonium Thiosulfate NH4+1 S2O3-2 These charges DON’T cancel each other out. NH4 Criss-cross the charges S2O3 (NH4)2S2O3 Final formula Notice that this time, the ammonium needs parentheses; there are two of them. The thiosulfate does NOT need parentheses; there is only one S2O3-2 ion. All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org, and it will be. Pirate Chemistry Transition Metals Thus far we have only dealt with ions that are in the main groups. These are groups I—VIII A on the periodic table. These are the main group elements: Charges +1 0 +2 +3 +/-4 -3 -2 -1 With few exceptions, these are easy to use. Remember, though, that we have been ignoring large sections of the periodic table. These are the transition metals. The transition metals are the elements in the middle of the Periodic Table. The reason that we have been ignoring them is that they get complicated. Most of the transition metals have the ability to go to more than one charge. For instance iron, Fe, can be either the Fe+2 charge or the Fe+3 charge. Copper, Cu, can be either the Cu+1 charge or the Cu+2 charge. Why does it matter? http://www.bbc.co.uk/schools/gcsebitesize/science/images/6_the_transition_metals.gif All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org, and it will be. Pirate Chemistry If Fe has a +2 charge, it will result in one formula. For instance: Fe+2 SO4-2 FeSO4 If Fe has a +3 charge, it will result in a different formula: Fe+3 SO4-2 Fe2(SO4)3 Notice that in the top example you end up with a different formula than the bottom example even though you are using the same atoms. FeSO4 and Fe2(SO4)3 are different substances as their pictures show: http://upload.wikimedia.org/wikipedia/commons/a/ab/Iron(II)-sulfate-heptahydrate-sample.jpg FeSO4 is a green crystal. http://img.alibaba.com/photo/244383957/Iron_III_sulfate.summ.jpg Fe2(SO4)3 is a yellow crystal. How do we deal with this? They both can’t have the same name, iron sulfate. To solve this, Chemists use a Roman Numeral to designate the charge on the transition metal ion. For example: Fe+2 Fe+3 iron (II) ion iron (III) ion Notice that the Roman numeral (II) matches the charge Notice that the Roman numeral (III) matches the charge Thus our problem is solved: Cation Anion Formula Name Fe+2 SO4-2 FeSO4 Iron (II) sulfate Fe+3 SO4-2 Fe2(SO4)3 Iron (III) sulfate Cu+1 O-2 Cu2O Copper (I) oxide Cu+2 O-2 CuO Copper (II) oxide Please note that the roman numeral does not usually match the number of the atoms present. In iron (II) sulfate there is only 1 Fe atom present, FeSO4. In copper (I) oxide, there are 2 Cu atoms present, Cu2O while in copper (II) oxide there is only 1 Cu present, CuO. The roman numeral tells us the charge on the ion, not the number of them present. All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org, and it will be. Pirate Chemistry Questions 1. Write the formula for the following ions: A. Acetate B. Bicarbonate C. Thiocyanate D. Peroxide E. Ammonium 2. Write the name of the following ions: A. CN-1 B. Cl-1 C. ClO3-1 D. SO4-2 E. PO3-3 3. Why are Roman numerals necessary for most transition metals? 4. Write the formula for the following ionic compounds: A. Calcium iodide B. Sodium oxide C. Potassium oxalate D. Ammonium hypochlorite E. Aluminum hydroxide F. Iron (III) phosphate G. Copper (II) nitrite H. Silver chloride I. Zinc cyanate 5. Write the name for each of the following ionic compounds: A. KCN B. (NH4)2O C. AlPO4 D. CaSO3 E. Na2CrO4 F. LiH2PO4 G. Mg(OH)2 H. Cu2CO3 I. Fe2(S2O3)3 J. ZnCl2 6. Each of these formulas has something wrong with it. Fix it: A. MgCl3 B. Na3SO4 C. CaOH2 D. Fe(III)Br3 E. Na+1I-1 F. OCa All text copyright Chris Smith 2009. All pictures obtained from internet and are copyright of their owners but assumed to be public accessible. If you are the owner of a picture and want it removed, email csmith@d211.org, and it will be.