Regents Chemistry: Atomic Structure Study Guide
advertisement

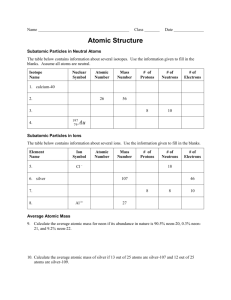
Regents Chemistry Atomic Structure Study Guide Complete the following: 1) Describe Rutherford's gold foil experiment and what he learned about atoms. 2) What are the relative masses, charges and locations of protons, electrons, and neutrons? 3) An atom has 7 protons and 8 neutrons. How many electrons does it contain? What element is this atom and what is it's isotopic name? What is its nuclear charge? 4) The atomic mass of an element is 39 atomic mass units (amu). It's atomic number is 19. What is the isotopic name of this element. How many protons, electrons and neutrons does it contain? What is its ground state configuration? What could be an example of its excited state configuration? 5) Why are atoms electrically neutral? What are isotopes? Given the isotopic symbols Aluminum-27 and Aluminum-28, list the correct number of subatomic particles for each. How would it differ from an isotope of Silicon-28? 6) Determine the number of protons, electrons, and neutrons in an atom of Sodium-23. What would be the number of protons, electrons, and neutrons in a +1 ion of sodium? Do the same for Chlorine-35 and -1 ion of chlorine. How do atoms differ from ions? 7) How do you determine the average atomic mass of an element if you know its relative abundance and the mass of each of its isotopes? 8) What is a bright line spectrum and how is it produced in terms of subatomic particles and energy?