Worksheet: Solubility Graphs Name______________
advertisement
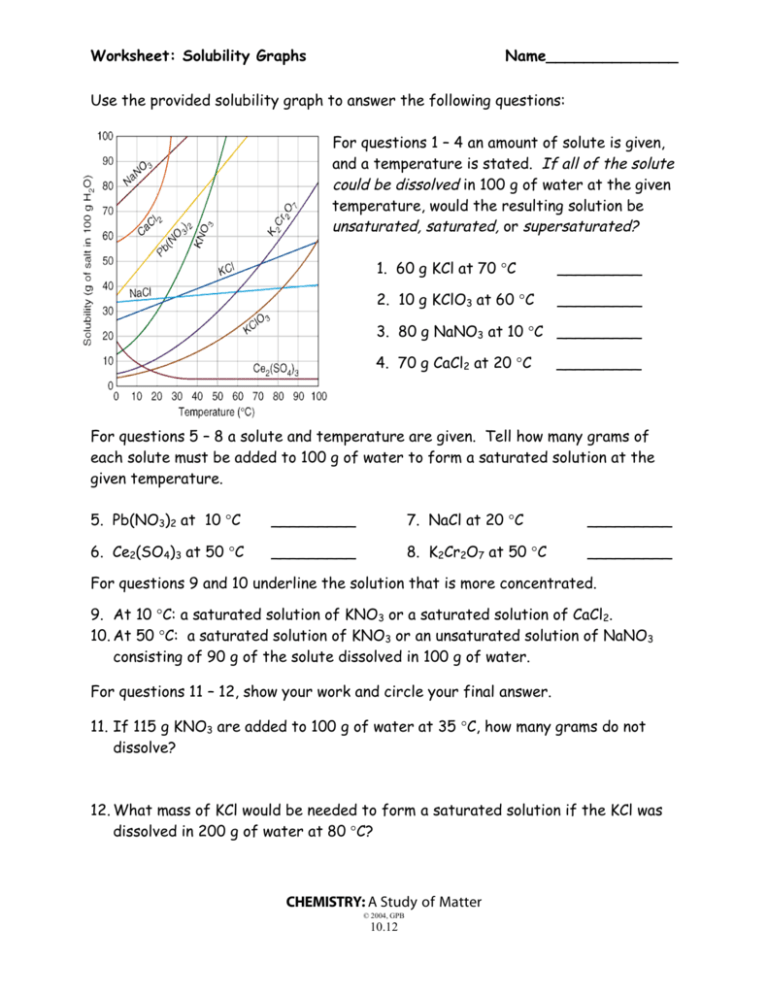
Worksheet: Solubility Graphs Name______________ Use the provided solubility graph to answer the following questions: For questions 1 – 4 an amount of solute is given, and a temperature is stated. If all of the solute could be dissolved in 100 g of water at the given temperature, would the resulting solution be unsaturated, saturated, or supersaturated? 1. 60 g KCl at 70 °C _________ 2. 10 g KClO3 at 60 °C _________ 3. 80 g NaNO3 at 10 °C _________ 4. 70 g CaCl2 at 20 °C _________ For questions 5 – 8 a solute and temperature are given. Tell how many grams of each solute must be added to 100 g of water to form a saturated solution at the given temperature. 5. Pb(NO3)2 at 10 °C _________ 7. NaCl at 20 °C _________ 6. Ce2(SO4)3 at 50 °C _________ 8. K2Cr2O7 at 50 °C _________ For questions 9 and 10 underline the solution that is more concentrated. 9. At 10 °C: a saturated solution of KNO3 or a saturated solution of CaCl2. 10. At 50 °C: a saturated solution of KNO3 or an unsaturated solution of NaNO3 consisting of 90 g of the solute dissolved in 100 g of water. For questions 11 – 12, show your work and circle your final answer. 11. If 115 g KNO3 are added to 100 g of water at 35 °C, how many grams do not dissolve? 12. What mass of KCl would be needed to form a saturated solution if the KCl was dissolved in 200 g of water at 80 °C? CHEMISTRY: A Study of Matter © 2004, GPB 10.12 Worksheet: More on Solubility Name______________ 1. Explain what is meant by the expression “like dissolves like”. 2. An unknown compound is observed to mix with benzene (a nonpolar solvent) but not with water. Is the unknown compound ionic or covalent? If the unknown compound is a liquid, will it be able to dissolve table salt?____________ Explain: 3. What are the chemical characteristics of a good dry-cleaning solvent? 4. Explain why you are more likely to overdose on vitamin A than on vitamin C. 5. Some industrial plants use water from nearby rivers and streams as a coolant. When the water is returned to the river or stream, the water is warmer than it was originally. This is referred to as “thermal pollution”. Using your knowledge of solubility, why might this thermal pollution be harmful to fish? CHEMISTRY: A Study of Matter © 2004, GPB 10.13 6. After a bottle of carbonated drink has been open for a while, it tastes “flat”. Explain why. 7. For most solid solutes, the degree of solubility in a liquid solvent (increases, decreases) with an increase in the temperature of the solvent. 8. Describe what happens to the degree of solubility of a gaseous solute in a liquid: a) with a decrease in the temperature of the solvent. b) with an increase in pressure ( ____________ Law). 9. The following statement is false: It is not possible to make a saturated solution from a substance that is described as only slightly soluble. Explain why this statement is false. CHEMISTRY: A Study of Matter © 2004, GPB 10.14 Lab: Solubility—Datasheet Name______________ Procedure: 1) Each lab group was assigned a different temperature, calculating the solubility of KCl in water at that temperature. Each group conducted the lab as seen on the video, and their data follows. You are to complete columns #5 and #6, using the provided data from each lab group. Data: #1 #2 #3 #4 Assigned Mass of Mass of Mass of Temperature evaporating evaporating evaporating dish + dish + dish + C cover cover + cover + KCl dry KCl solution 10 20 30 40 50 60 70 80 90 37.81 g 37.65 g 36.95 g 37.80 g 36.50 g 37.75 g 35.98 g 37.81 g 36.99 g 48.75 g 50.42 g 47.82 g 48.19 g 47.49 g 50.08 g 48.29 g 48.64 g 48.96 g #5 Mass of KCl #6 Mass of water (#4 - #2) (#3 - #4) #7 Mass of KCl per 100 g of water 40.54 g 40.99 g 39.81 g 40.63 g 39.75 g 41.52 g 39.86 g 41.37 g 40.98 g 2) Column #7 is to be calculated as to express the solubility of KCl in grams per 100 grams of water. Following the equation given, complete column #7 for each group’ s data. Mass of KCl ? g KCl = 100 g H2O = Mass of water CHEMISTRY: A Study of Matter © 2004, GPB 10.10 3) Construct a graph using the vertical axis for grams of solute per 100 g of solvent and the horizontal axis for temperature. Staple your graph to this paper. BE SURE TO USE PROPER SCIENTIFIC GRAPHING TECHNIQUES. Conclusion Questions: 1. The solubility of a solute is the maximum mass of the solute that will dissolve in a certain amount of water at a certain ______________. This is the same as saying that solubility is the concentration of a (unsaturated, saturated) solution of the solute. 2. From your graph, what mass of KCl can be dissolved in 100 g of water at these temperatures? Use dotted lines on your graph to show how you used your graph to determine your answers: a) 25 C__________________ b) 55 C____________________ 3. For each of the following, tell whether the solution would be saturated, unsaturated, or *crystallizing. (Hint: Plot the point and see whether it lies above, below, or on the best-fit line. Remember that the line represents a ___________ solution.) a) 40.0 g of KCl in 100 g of water at 75 C b) 34.0 g of KCl in 100 g of water at 55 C c) 45.0 g of KCl in 100 g of water at 25 C ___________________ ___________________ ___________________ *Normally, crystallization (rather than supersaturation) occurs when more solute is present than what can be dissolved in a given amount of solvent at a given temperature. Crystallization simply refers to the excess solute “ crystallizing”and settling out of the solution. Supersaturation is rare. CHEMISTRY: A Study of Matter © 2004, GPB 10.11








