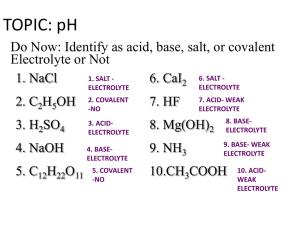
Logs and pH pH =
advertisement

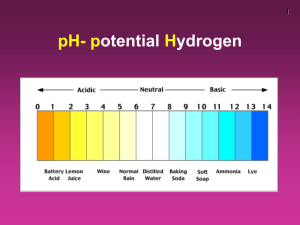
Logs and pH Knowing now that the pH scale is a logarithmic scale, we can perhaps better understand the shape of the titration curve we saw in the previous experiment. Each change of 1 on the pH scale is really a change of 10 so the concentration of acid at a pH of 7 is 106 or 1,000,000 times less than at a pH of 1. Those tiny concentrations mean that each drop of the titration will cause a massive change in pH values. The graph then levels off as the concentration of base becomes higher and higher so each drop matter less and less. 14 Base has accumulated. Acid is neutralized. Tiny acid concentration. Base has major effect. Base starts to accumulate. pH 7 High acid concentration. Base has little effect. 0 Volume of NaOH added How, then, do we go from these numbers to a convenient value on the pH scale? What is the relationship between logs and pH? The pH scale is the negative logarithm of the concentration of the acidic substance in a solution, H+. + pH = - log [H ] Or put another way, the pH scale tells us approximately how many zeroes are present in the value or how many places we have to move the decimal point to get to our concentration. Notice how at a pH of 1 the concentration of the H+ is 0.1 or 1x10-1. Notice that at a pH of 14 the concentration is a much smaller value, 1x10-14. The reason that the more acidic solution has the smaller pH value is because the pH is really measuring the negative exponent. The more negative the value becomes, the smaller the concentration. The pH values here are really telling us the negative exponent of the concentration! H+ (decimal) pH 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 1 0.1 0.01 0.001 0.0001 0.00001 0.000001 0.0000001 0.00000001 0.000000001 0.0000000001 0.00000000001 0.000000000001 0.0000000000001 0.00000000000001 pH 5 14 H+ (decimal) 0.00001 0.00000000000001 H+ (sci. notation) 1x100 1x10-1 1x10-2 1x10-3 1x10-4 1x10-5 1x10-6 1x10-7 1x10-8 1x10-9 1x10-10 1x10-11 1x10-12 1x10-13 1x10-14 H+ (sci. notation) 1x10-5 1x10-14 This also works for numbers in-between the whole number pH values. Look: Notice how every change in 1 pH value means that the negative exponent increases by 1 or the decimal gets bigger by 1 place or a factor of 10. pH [H+] sci [H+] decimal 1.00 1x10-1 1.30 5x10 -2 0.05 2.00 1x10 -2 0.01 2.10 8x10-3 0.008 2.22 6x10 -3 0.006 2.40 4x10-3 0.004 2.70 2x10 -3 0.002 3.00 1x10 -3 0.001 3.05 9x10-4 0.0009 3.10 8x10 -4 0.0008 3.15 7x10-4 0.0007 3.22 6x10 -4 0.0006 3.30 5x10 -4 0.0005 3.40 4x10-4 0.0004 3.52 3x10 -4 0.0003 3.70 2x10-4 0.0002 4.00 1x10 -4 0.0001 4.05 9x10 -5 0.00009 4.10 8x10-5 0.00008 4.15 7x10 -5 0.00007 4.22 6x10-5 0.00006 4.30 5x10 -5 0.00005 4.40 4x10 -5 0.00004 4.52 3x10-5 0.00003 4.70 2x10 -5 0.00002 5.00 1x10-5 0.00001 5.05 9x10 -6 0.000009 5.10 8x10 -6 0.000008 5.15 7x10-6 0.000007 5.22 6x10 -6 0.000006 5.30 5x10-6 0.000005 5.40 4x10 -6 0.000004 5.52 3x10 -6 0.000003 5.70 2x10-6 0.000002 6.00 -6 0.000001 1x10 0.1 pH Calculations If you want the pH of a solution all you have to do is take the negative logarithm of the [H+]: pH = - log [H+] [H+] 1 x10-5 6 x10-3 4 x10-13 pH -log (1 x10-5) = 5 -log (6 x10-3) = 2.22 -log (4 x10-13) = 12.4 If you want to do the reverse, meaning you have the pH and you want the [H+], then you have to perform the anti-log which is 10x. [H+] = 10-pH pH 3.6 13.4 0.7 [H+] 1x10-3.6 = 2.51 x10-4 1x10-13.4 = 3.98 x10-14 1x10-0.7 = 0.200 or 2.00 x10-1 pOH Scale So the pH scale measures how acidic a solution is but water has two parts to it, H+ and OH-1. One part acid, one part base. As a solution gets more and more acidic, it gets less and less basic. Or to put another way, as the solution gets more and more basic, it gets less and less acidic. There is no reason that we couldn’t measure the acidity or basicity of a solution by measuring the OH-1 content. To do so, we use the exact same ideas as before except now we would be looking for pOH: -1 pOH = - log [OH ] pOH is the exact opposite of pH. As the pOH goes down, the solution becomes more basic, as the pOH goes up, the solution becomes more acidic. Acids Bases Neutral pOH Calculations These are done the exact same way as for pH [OH-1] 3 x10-3 9 x10-6 1 x10-11 pOH -log (3 x10-3) = 2.52 -log (9 x10-6) = 5.05 -log (1 x10-11) = 11 If you want to do the reverse, meaning you have the pOH and you want the [OH-1], then you have to perform the anti-log which is 10x. [OH-1] = 10-pOH pOH 2.9 10.6 2.5 [OH-1] 1x10-2.9 = 1.26 x10-3 1x10-10.6 = 2.51 x10-11 1x10-2.5 = 3.16 x10-3 pH Square Look at the diagram on the previous page. What do you notice about each pH and pOH value. What is the pOH when the pH is 7? It’s 7. What is the pOH when the pH is 2? It’s 12. In fact: pH pOH 0 14 1 13 2 12 3 11 4 10 5 9 6 8 7 7 8 6 9 5 10 4 11 3 12 2 13 1 14 0 Notice any relationship between these two columns? The pH and the pOH add up to 14! pH pOH pH + pOH 0 14 14 1 13 14 2 12 14 3 11 14 4 10 14 5 9 14 6 8 14 7 7 14 8 6 14 9 5 14 10 4 14 11 3 14 12 2 14 13 1 14 14 0 14 pH + pOH = 14 Adding this information to the relationships we saw before: pH = - log [H+] [H+] = 10-pH pOH = - log [OH-1] [OH-1] = 10-pOH pH + pOH = 14 We can summarize all these equations on one diagram called the pH square: -log [H+] + [H ] pH 1x10-pH 1x10-14/[OH-1] 1x10-14/[H+] -1 [OH ] 14-pH 14-pOH -log [OH-1] pOH 1x10-pOH You should be able to see that if given any one piece of information, pH, pOH, [H+], or [OH-1], you should be able to get all of the other pieces of information relatively quickly. For instance: Given: [H+] = 1 x10-5 Knowing this, you can easily get the pH: -log 1x10-5 = 5 Knowing that the pH is 5, the pOH can easily be obtained because pH + pOH = 14 so pOH = 9 Knowing that the pOH is 9, the [OH-1] can easily be obtained: 1x10-pOH = 1x10-9 [H+] pH pOH [OH-1] 1x10-3 3 11 1x10-11 5x10-8 7.3 6.7 2x10-7 8x10-5 4.1 9.9 1.26x10-10 Questions 1. For each of the following concentrations of [H+] , calculate the pH: a) 3.28 x10-4 b) 9.43 x10-13 c) 2.71 x10-8 d) 1.00 x10-3 e) 1.00 x10-12 2. For each of the following pH values, calculate the concentration of [H+]: a) pH = 5 b) pH = 3 c) pH = 2.8 d) pH = 13.7 e) pH = 6.9 3. For each of the following pOH values, determine whether the solution is acidic, basic, or neutral: a) pOH = 4.5 b) pOH = 9.2 c) pOH = 7 d) pOH = 13.8 e) pOH = 0.4 4. For each of the following concentrations of [OH-], calculate pOH: A) 6.32 x10-2 B) 9.28 x10-9 C) 7.56 x10-3 D) 1.00 x10-5 E) 1.00 x10-11 5. For each of the following pOH values, calculate the concentration of [OH-1]: A) pOH = 3.5 B) pOH = 9.1 C) pOH = 4.6 D) pOH = 2.4 E) pOH = 7.0 6. Using the pH square, fill in the blanks on the following chart: [H+] pH pOH [OH-1] 1.0 x10-4 3.9 1.2 8.3 x10-8