pH and pOH
advertisement

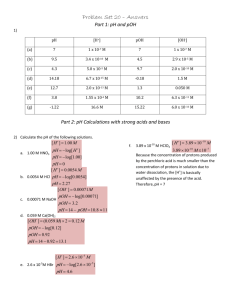
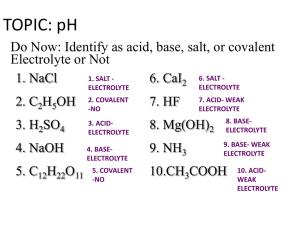
*Name the following acids: *HI *HNO3 *HCl *Write the formula for the following acids: *Hydrofluoric Acid *Nitrous Acid *Hydrobromic acid * * Unit 10, Day 2 Kimrey 17 May 2013 *Identify strong and weak acids *Calculate the pH and pOH of an acid given a concentration. *Determine the concentration of an acid given a pH or pOH. * *There are two strengths of acids: strong and weak. *Strong acids completely dissociate (break up) in water. *Weak acids do not completely dissociate. *There are 6 strong acids. *HCl *HBr *HI *H2SO4 *HNO3 *HClO4 * *pH is a logarithmic scale that measures the concentration of the [H+] ion in solution. *Goes from 0 -14 * 0 - 6.99 is acidic *14 – 7.01 is basic *pH = -log[H+] *[H+]=10-pH * *Don’t freak over logs and anti-logs, your calculator does them for you! *Ex. Find the pH of a HCl solution with a H+ concentration of 1 x 10-6. *pH = -log[H+] *pH = 6 * *When acids break up, the concentration of hydrogen is sometimes explained by using hydronium *Hydronium is H3O *Water + Hydrogen *Just do math the same way! * *Find the pH of a 0.03 M solution of HBr. *pH = -log[H+] *pH = 1.5 * *What is the concentration of a HCl solution that has a pH of 3? *[H+]=10-pH *[H+] = .001M * *Same as pH, but opposite. *Goes from 0 -14 * 0 - 6.99 is basic *14 – 7.01 is acidic *pOH = -log[OH-] *[OH-]=10-pOH * *Calculate the pOH for a solution of NaOH with a concentration of 1 x 10-4 M. *pOH = -log[OH-] *pOH = 4 * *Calculate the pOH for a solution of KOH with a concentration of 0.45 M. *pOH = -log[OH-] *pOH = .35 * *What is the concentration of a solution the has a pOH of 13.5? *[OH-] = 10-pOH *[OH-] = 3.16 x 10 -14 M * *Remember that both the pH and pOH scale go from 0-14. *As the concentration of [H+] or [OH-] goes up the other must go down. *These two relationships allows us to assume that: * pH + pOH = 14 * *What is the pH of a solution that is found to have a pOH of 10? *pH = 4 * *What is the pH of a .067 M solution of LiOH? *pOH = 1.17 *pH = 12.8 * *What is the pOH of a 0.0056 M solution of HCl? *pH = 2.25 *pOH = 11.75 * *What is the pH of a solution HI with a concentration of .0089 M? *What is the concentration of a strong acid with a pH of 3.45? *What is the pH of a solution of a NaOH with a concentration of 5.67 x 10-4 M? *









![[H + ] [OH ] - CCBC Faculty Web](http://s2.studylib.net/store/data/005793401_1-b043355121eb738cc68e8c8b1b02be73-300x300.png)