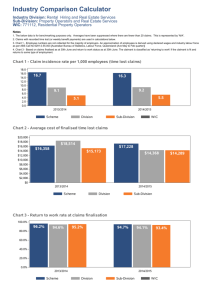
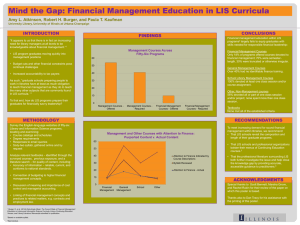
laboratory information system integration
advertisement

LABORATORY INFORMATION SYSTEM INTEGRATION: A SURVEY OF CURRENT AND PROPOSED LOCAL OR REGION MODELS OF CONNECTIVITY A Report Prepared By: The Ontario Hospital e-Health Council Hospital Laboratory Information Systems Advisory Group November 2006 Table of Contents 1. EXECUTIVE SUMMARY ......................................................................................... 1 2. INTRODUCTION AND BACKGROUND.................................................................. 2 3. RESTRUCTURING & HEALTH SYSTEM TRANSFORMATION INITIATIVES: IMPACT ON LABORATORY INFORMATION SYSTEMS ..................................... 4 3.1. Laboratory Restructuring: Ontario Regional Laboratory Services Planning (ORLSP) ........................................................................................................................4 3.2. Ontario Laboratory Information System (OLIS) .......................................................6 3.3. Local Health Integration Networks (LHINs) ..............................................................7 4. SURVEY OF CURRENT & PROPOSED LIS INTEGRATION SOLUTIONS ........... 9 4.1. Environmental Scan Methodology ............................................................................9 4.1.1. Inquiry Approach ...........................................................................................9 4.1.2. Constructing the Interview Questionnaire......................................................9 4.2. The Selection Process ..............................................................................................10 4.3. Transforming the Results into Profiles ...................................................................10 4.4. Follow-Up ...................................................................................................................10 5. MODELS OF INTEGRATION ................................................................................ 11 5.1. Summary of Models of Integration ..........................................................................11 5.1.1. Strategic Decision-Making Considerations..................................................11 5.2. OLIS & LIS Integration .............................................................................................13 5.2.1. Interfacing....................................................................................................13 5.2.2. Nomenclature ..............................................................................................13 5.3. LHINs & LIS Integration ............................................................................................13 5.3.1. Governance .................................................................................................14 5.3.2. Finance........................................................................................................14 6. CRITICAL SUCCESS FACTORS.......................................................................... 15 7. BENEFITS ............................................................................................................. 15 8. ACKNOWLEDGEMENTS...................................................................................... 16 9. APPENDIX A: ANALYTICAL SUMMARY OF ENVIRONMENTAL SCANS ......... 17 10. APPENDIX B: FINAL ENVIRONMENTAL SCAN QUESTIONNAIRE ................. 20 11. APPENDIX C: FINAL ENVIRONMENTAL SCAN PROFILES .............................. 24 11.1.Central East 1 ...........................................................................................................24 11.2.Central West .............................................................................................................28 11.3.East 2.........................................................................................................................30 11.4.Eastern Ontario Regional Laboratory Association ...............................................33 11.5.electronic Child Health Network .............................................................................36 11.6.Grey Bruce Health Services ....................................................................................40 11.7.Hamilton Regional Laboratory Medicine Program................................................43 11.8.InterHospital Laboratory Partnership ....................................................................46 11.9.London Laboratory Services Group.......................................................................49 11.10.Mount Sinai Hospital..............................................................................................52 11.11.North Eastern Ontario Network ............................................................................55 11.12.Northwest Health Network ....................................................................................58 11.13.Pathology Information Management System ......................................................61 11.14.Sunnybrook Health Sciences Centre ...................................................................65 11.15.Toronto Medical Laboratories...............................................................................68 11.16.Trillium Health Centre............................................................................................71 11.17.William Osler Health Centre ..................................................................................74 1. EXECUTIVE SUMMARY Ontario’s health care environment is changing rapidly, not only with the implementation of the Local Health Integration Networks (LHINs), but also with a push towards a comprehensive electronic health record (EHR). These twin processes of regional and information technology transformation are particularly important for laboratory medicine, where the ability to share laboratory information and results across multiple care providers has long been deemed an essential component for the delivery of quality patient care. Laboratory medicine services are rapidly expanding and increasing in complexity as a result of advances in technology and the increasing acuity and demands of an aging population. This “growth” in laboratory medicine, along with the implementation of regional laboratory system models developed through the Ontario Regional Laboratory Services Planning (ORLSP) initiative, will increase the demand for integration of Laboratory Information Systems (LIS) between hospital laboratories and their partners in the coming years. Laboratory Information Systems (LISs) are the infrastructure backbone for clinical laboratory operations. Their functionality extends beyond the simple transmission of orders and results. LISs manage the processes of the laboratory including: communication of specimen collection, storage and transportation requirements, specimen identification and tracking including directing specimens to appropriate workstations within the laboratory and identifying those tests which are referred out to reference laboratories. LIS functionality between participating laboratory service providers (including hospital laboratories) in a regional laboratory system must be seamlessly integrated to support key processes including: specimen management, identification and immediate electronic transmission of orders and results between laboratories The Ontario Hospital Association’s (OHA) Hospital e-Health Council tasked its Hospital Laboratory Information Systems Advisory Group (HLISAG) to study regional laboratory service models in the hospital sector beginning in the summer of 2005. The goal was to gather information on existing and planned models of LIS connectivity and integration, including data exchange processes, governance and finance. The resulting report is intended to provide hospital and regional health care executives with an overview of models and options, identify key benefits and critical success factors of existing projects, and leverage the wealth of expertise that exists in Ontario to enhance future plans for LIS connectivity and integration. This report identifies four main models of LIS integration that have been used by organizations, each supporting their specific needs. Capital and operating cost considerations were recognized as major drivers in model selection. Among the regional initiatives surveyed, the identified benefits included: reduced turnaround time, reduced test duplication, decreased transcription errors, improved access to patient information and a reduction in time-consuming manual processes. Critical success factors identified included: laboratory medical, scientific and technical staff participation, ensuring that benefits are shared with all partners and the ability to maximize existing technical solutions. This report also notes that regional projects will need to have an increasing awareness of the role of the Ontario Laboratories Information System (OLIS) and LHINs, but that these initiatives are best viewed as enhancements to projects, and will not replace the need for regional LIS integration and connectivity initiatives. 1 2. INTRODUCTION AND BACKGROUND e-Health is a major component of health care innovation in Ontario and a key element of the Ministry of Health and Long-Term Care’s (MOHLTC) health system transformation agenda. A comprehensive electronic health record (EHR) is an essential enabler to the creation of a patient-centered health care system. It is crucial to achieving seamless quality care from outpatient primary care to inpatient tertiary and quaternary care in Academic Health Science Centers. According to Canada Health Infoway (CHI), laboratory information is a major component of the EHR and accounts for a significant proportion of the over 2000 transactions that occur every minute in health care in Canada 1 . In Ontario, laboratory services are delivered through four distinct providers: a) hospital-based clinical laboratories, b) community-based (“private”) laboratories, c) physician offices (restricted menu of tests provided only by a physician to their own patients) and d) public health laboratories. Almost all hospital and private laboratories utilize Laboratory Information Systems (LIS) not only to collect and store laboratory results but also to manage and support the operations and business practices of the laboratories. Laboratory services are tightly regulated in Ontario; individual laboratories and specimen collection centers must be licensed by the MOHLTC-Laboratories Branch and meet the rigorous standards of the Ontario Laboratory Accreditation (OLA) program. The importance of LISs in the delivery of quality laboratory services is emphasized by the inclusion of 50 different LIS standards and/or recommended guidelines in the OLA requirements. Despite the critical role LISs play in the delivery of laboratory medicine services, to date there has been little, if any, standardization of the structure and functionality of these systems. The situation is further complicated by the required integration between LIS and other clinical, financial and data systems in hospitals. Ontario hospitals have used varied strategic approaches to internal information system integration. Many have opted to purchase single vendor comprehensive “suite” systems while others have adopted “best of breed” strategies, using foreign system interfaces to integrate multiple systems from different vendors (see Figs. 1a and 1b). Single Vendor Health Information System Platform Patient Management Laboratory Radiology Pharmacy Integrated Modules Fig. 1a: Generic Depiction of Single Vendor “Suite” of Systems 1 Canada Health Infoway. End User Acceptance Strategy: Current State Assessment. May 5, 2005. 2 Patient Management System Interface Engine Interface Engine Interface Engine Laboratory Information System Radiology Information System Pharmacy Information System Fig. 1b: Generic Depiction of “Best of Breed” Systems Until recently, the functionality, configuration and integration of hospital LISs with other clinical and financial systems has largely been internally focussed within individual hospital corporations. However, three major health transformation initiatives are shifting the focus to regional and provincial connectivity and integration of LISs: i) Ontario Regional Laboratory Services (ORLSP) planning initiative ii) Local Health Integration Networks (LHINs) iii) Ontario Laboratory Information System (OLIS) This report by the Ontario Hospital Association (OHA) Hospital Laboratory Information Systems Advisory Group (HLIS AG) provides an overview of the impact and relationship of these major restructuring initiatives on hospital LISs and a survey of current and planned solutions. 3 3. RESTRUCTURING & HEALTH SYSTEM TRANSFORMATION INITIATIVES: IMPACT ON LABORATORY INFORMATION SYSTEMS 3.1. Laboratory Restructuring: Ontario Regional Laboratory Services Planning (ORLSP) Since the Laboratory Services Restructuring initiative in the early 1990s to the more recent ORLSP project, clinical laboratories in Ontario have been the subject of almost continuous study and restructuring planning. Throughout the 1990s the principle driver behind laboratory restructuring was the search for financial efficiencies. However, over the last few years, strategic planning for laboratories has shifted to focus on three additional key issues: i) Shortages of laboratory staff, including technical, management and medical/scientific personnel, ii) Increasing demands for new tests and technologies iii) Ensuring laboratory medicine services meet the quality standards of OLA In response to these new challenges, representatives of hospital laboratories (OHA), physicians (Ontario Medical Association, OMA), the community laboratories (the Ontario Association of Medical Laboratories, OAML), and the MOHLTC developed an agreement in May 2000 for a regional planning approach to laboratory services delivery. The ORLSP initiative was established by the MOHLTC to bring together representatives of laboratory providers in each of nine planning regions to develop a coordinated regional laboratory services system. A Steering Committee for each region was established with members appointed by the OHA, OMA and OAML. Each region was responsible for preparing a laboratory services plan, taking into account all of the services required for the region, and ensuring all options for service delivery were fully explored. Guiding principles for the planning initiative included: I. Each region would develop a laboratory services plan that: • Included hospital and community lab services • Ensured accessibility • Limited duplication of operations and services • Met quality standards • Ensured an appropriate critical mass where testing is performed. II. Funding systems would allow funding to follow service. III. Consistent structures would be established in each region to oversee access to services and quality standards. IV. Where testing could be centralized, it would occur in an orderly manner. V. A laboratory physician and scientist human resources (HR) plan would be established to ensure availability of expert consulting and services. VI. An accountability framework would be created for each region to ensure consistent tracking and reporting of statistical and financial information. VII. Contractual arrangements, with obligations for quality and service delivery, would be implemented for each service provider. 4 The ORLSP reports identified many opportunities for the consolidation and sharing of laboratory services between hospital laboratories. However, throughout the ORLSP discussions, in every region, connectivity and integration of LISs was identified as one of the most important enablers for the successful implementation of regional laboratory service delivery models. As previously mentioned, LISs are the infrastructure backbone for clinical laboratory operations. Their functionality extends beyond the simple transmission of orders and results. LISs manage the processes of the laboratory including: • Communication of specimen collection • Storage and transportation requirements • Specimen identification to support automated instrumentation (e.g. bar coding, container identification and differentiation, aliquoting) • Specimen tracking, including directing specimens to appropriate workstations within the laboratory and identifying those tests which are referred out to reference laboratories • Collecting workload and volume data (by patient type and by clinical area or client) • Supporting reflex testing and test utilization management • Monitoring turnaround time • Documenting required action of laboratory staff (e.g. communication of critical results) • Supporting workload management by tracking specimens awaiting • Facilitating the transfer of workload from one workstation to another, where necessary LIS functionality between participating laboratory service providers (including hospital laboratories) in a regional laboratory system must be seamlessly integrated to support key processes including: specimen management (standardization of collection, tracking during storage and transportation between laboratories), identification (bar code commonality and recognition) and immediate electronic transmission of orders and results between laboratories (see Figure 2). To date, some regions of the province have made limited progress towards connecting and integrating hospital LISs to support the regional laboratory service delivery models (as evident in the survey results illustrated in Section 4 of this report). More extensive integration has been impeded by funding constraints, the technical challenges of integrating multiple legacy LISs and limited availability of laboratory and Information System/Information Technology (IS/IT) staff resources. In addition, the Ontario Laboratory Information System (OLIS) initiative and the LHINs have been established and both require significant consideration in the strategic planning around regional integration of LIS. 5 Client Lab Hospital Client Lab Hospital Clinical Area Clinical Area results orders orders rerouted orders Client Lab specimens specimens Reference Lab Hospital results rerouted orders Reference Lab Client Lab results results specimens specimens Fig. 2: Generic depiction of exchange of orders and results and movement of specimens between laboratories in a regional laboratory model. 3.2. Ontario Laboratory Information System (OLIS) The idea of a province-wide laboratory information system was originally envisaged in the early 1990s. Following more than 10 years of discussions and planning, implementation of OLIS officially began in 2005 with the selection of the system vendor (Capgemini Canada Inc.) and an invitation to hospital and community laboratories to apply to become “early adopters”. OLIS aims to be an integrated, province-wide system for the electronic exchange of laboratory information among authorized practitioners (physicians, nurse practitioners, midwives, dentists, Public Health Units), laboratories (hospital, community and public health laboratories) and the MOHLTC. It is important to note that OLIS is not a replacement for LIS, hospital information system (HIS) or clinical management systems (CMS), and will not be a substitution for LIS integration in regional laboratory service delivery models. Its primary role will be to serve as a vehicle for the transfer of laboratory orders (e.g. from an ordering community based physician to a provider laboratory) and a repository of laboratory results (from all laboratory providers in the province) (See Figure 3). 6 Practitioners Order Result Submission Query Hospitals Query Order Result Submission Query Query Order Response Order Retrieval Result Retrieval Query Response Result Submission Query Public Health Labs Query Response Order Retrieval Order Retrieval Result Retrieval Query Response OLIS Clinical Repository Order Order Retrieval Community Labs Order Retrieval Specimen Collection Centres Order MOHLTC, Planners, Researchers Report Request Report Response Phase I Pseudonymous Repository Phase II OLIS Orders Repository Fig. 3: High-level depiction of OLIS functionality and relationships As of February 2006, Smart Systems for Health Agency (SSHA) completed a major milestone in connecting all public hospitals in Ontario to the managed private network (MPN), thereby providing all hospitals with the ability to securely exchange and share electronic health information related to e-Health initiatives, including SSHA-hosted solutions like OLIS. On behalf of OLIS, SSHA is working with community laboratories and their main corporate sites where the laboratory information systems reside, to connect community laboratories to the MPN and enabling the secure sharing of laboratory information. The provincial e-Health strategy includes establishing SSHA network connectivity to all hospital and community medical laboratories. 3.3. Local Health Integration Networks (LHINs) In 2004, the MOHLTC announced that LHINs will be responsible for planning, integrating and funding local health services. There are 14 LHINs with specific geographic boundaries. The LHINs will be responsible for the following health care providers: • • • • • • Hospitals Divested psychiatric hospitals Community Care Access Centres Community Support Service Organizations Community Mental Health & Addictions Agencies Community Health Centres • • • • • • 7 Long-Term Care Homes Public Health Physicians Ambulance Services (emergency & non-emergency) Laboratories Provincial networks and programs On March 1, 2006, the government of Ontario passed The Local Health System Integration Act, 2006. This legislation defines the roles, responsibilities, accountability and governance of the LHINs. As the LHINs move forward with the development of Integrated Health Service Plans (IHSPs) there will be increasing pressure to integrate and connect health care information systems across providers throughout each LIHN. For hospital clinical laboratories, there will likely be significant differences between the nine original regional laboratory plans developed under the ORLSP process and the model of laboratory services which will evolve through the 14 LHIN IHSP processes. Regardless of the final models for laboratory service delivery, it is clear that the integration of LISs will be an essential enabler for health care transformation. 8 4. SURVEY OF CURRENT & PROPOSED LIS INTEGRATION SOLUTIONS Laboratories and LIS are essential components of the health care system. As restructuring and transformation of the health care system proceeds, it is clear that LIS integration must be included in the strategic planning processes. The Hospital Laboratory Information Systems Advisory Group (HLISAG) recognized that information on current and planned regional laboratory data sharing and LIS integration solutions would provide valuable information for strategic planning and decision-making for the on-going implementation of regional laboratory systems and the LHINs. To this end, the HLISAG initiated an Environmental Scan. 4.1. Environmental Scan Methodology The goal of the Environmental Scan was to create an inventory document that identified: • Current or planned lab data exchange initiatives/models • Details of models including: LIS vendors involved, governance models, relationships to laboratory operational models, funding, key success factors, approach to Quality Assurance (QA) • Planned approach to OLIS • Key contacts 4.1.1. Inquiry Approach A standard list of specific questions was developed to capture the key elements of each regional laboratory information integration model, such as: • Purpose • Transactional model details • Data shared • Stakeholder involvement • Administrative control model • Funding model • Standards • QA • Privacy and security • Critical success factors • Best practice outcomes The intended outcome of the environmental scan was a compilation and comparison of responses from each region to illustrate key areas of commonality and difference between each regional LIS integration and connectivity initiative. The actual scan was conducted in the form of a guided interview with the key contact for each project/initiative. 4.1.2. Constructing the Interview Questionnaire The HLISAG utilized the expertise within its membership (see Section 8 for membership list) to conduct an iterative writing process. Once the target areas for further exploration were identified, members worked collaboratively to develop specific questions. A series of meetings were held to establish the initial set of questions. 9 The preliminary series of questions was tested in guided interviews at two pilot sites: Central West - West Node Microbiology and the North Eastern Ontario Network (NEON). Both pilot sites were initiatives represented by HLISAG members and fit the selection criteria (outlined below). Pilot feedback was analyzed and the Environmental Scan questionnaire revised (see Appendix B: Final Environmental Scan Questionnaire). 4.2. The Selection Process The group selected to complete the questionnaire was not tasked with providing a comprehensive inventory of all LIS integration and connectivity projects in Ontario but was designed to be representative of the different laboratory regions and diverse hospital sizes. The impact of OLIS early adoption projects at hospital sites was also taken into account and included in the selection. The final selected group included representation from: • 17 total initiatives • 2 province-wide initiatives • All 5 OHA regions • All 9 ORLSP regions • Small, Community and Teaching Hospitals • All 4 OLIS early adopter hospitals A complete summary of the selected group can be found in Appendix A: Analytical Summary of Environmental Scan Profiles. 4.3. Transforming the Results into Profiles Once results from the Environmental Scan were collated and analyzed by HLISAG members, answers from the guided interviews were transferred to a table for comparison. Four categories were developed to describe the overall integration and connectivity models and to facilitate evaluation: • Multi-Vendor, Multi-System (completely separate systems and LIS databases, with distributed control, but interfaced to each other) • Single-Vendor, Multiple-System (multiple LIS instances of a single vendor solution, connecting via interface engines) • Single-Vendor, Single-System (complete integration of lab systems) • Province-wide Data Repository (interfacing multiple LISs to a common provincial database, providing access to that data via a portal) 4.4. Follow-Up To support the descriptions of the above integration and connectivity models and to identify key strategic decision making rationale, two areas for follow-up work were identified: • Diagrams were created to complement each profile and, where necessary, show the differences between variations within models • In-depth interviews were conducted at selected sites, focusing on the strategic decisions made in establishing regional LIS connectivity and integration initiatives Queries were made with representatives of each of the major categories. 10 5. MODELS OF INTEGRATION Historically, the factors which drove the selection of one LIS integration model over another were largely related to laboratory business operations. “Point to point” connectivity approaches were common between laboratory providers, with the primary focus of transmitting orders and results. As health care transitions to EHR’s, strategic decision-making for LIS integration has increased in complexity in terms of model options and factors for consideration. 5.1. Summary of Models of Integration Detailed Environmental Scan information for each initiative surveyed and profile created can be found in Appendix C: Final Environmental Scan Profiles. Analysis of the data revealed four distinct models of LIS integration: A) Single vendor with a single LIS platform serving multiple sites B) Single vendor with multiple LIS platforms integrated/connected through an interface engine C) Multiple vendors with multiple LIS platforms integrated/connected through one or more interface engines D) Interface to a province-wide data repository. 5.1.1. Strategic Decision-Making Considerations In the course of research for this report, the decision-making processes involved in selecting each model of integration were studied. Certain considerations tended to drive the selection of one model over the others, and frequently depended on the environmental situation existing prior to integration. This section is a compilation of the key issues that were identified in the decision-making process. Although province-wide interfacing projects such as electronic Child Health Network (eCHN) and Pathology Information Management System (PIMS) were studied, strategic decision-making processes for such integration models were considered separate from regional lab services sharing, and thus are not studied in detail in this section. 5.2.1.1 Single Vendor-Single LIS Platforms a) Operational and Implementation Considerations: • Facilitates and supports regional standardization of laboratory policies, procedures and processes. Facilitates inter-site rotation of laboratory staff. • Requires strong governance and regional decision-making for implementation and operation. Individual sites must forfeit some or all of their autonomy. 11 • • Single LIS places entire regional laboratory system at high risk for unscheduled “downtime” or system failure. Requires single nomenclature standard for region. b) Financial Considerations: • Requires significant initial investment (e.g. $6-10 million). • Requires regional Enterprise Master Patient Index (EMPI). • Requires restructuring of existing LIS-HIS/ADT integration at each site (unless single IS suite system is implemented throughout region). • Once implemented, on-going operating costs are less than multiple vendor/system models. • Minimally leverages existing LIS investments. Highly advantageous for regions with a large number of legacy systems, which require replacement. 5.2.1.2 Single Vendor-Multiple LIS Platforms a) Operational and Implementation Considerations: • Does not require costly re-engineering of LIS-HIS/ADT and other system integration at each site. • Requires development of interface solution compatible with multiple versions of the same software. • Allows for phased-in approach to further integration. b) Financial Considerations: • Leverages existing LIS investments. • Controls cost of upgrading exiting LIS installations. • Operational resources focused on maintaining the interface engines. 5.2.1.3 Multiple Vendors-Multiple LIS Platforms a) Operational and Implementation Considerations: • Requires minimal restructuring of current laboratory business practices. Allows each laboratory site to maintain current structures, policies and procedures. • Autonomy of individual sites and institutions maintained. • Easiest to implement in absence of regional governance or decision making authority. • Individual site LISs function independently, failure of one LIS does not affect core laboratory services at all sites. • Utilizes existing LIS implementations, existing interface solutions and available technical expertise at hand. b) Financial Considerations: • Does not require costly re-engineering of LIS-HIS/ADT and other system integration at each site. • May be phased in throughout a region depending on each site’s readiness and business case (e.g. implementation prioritized by order and result transaction volumes). • Highly scaleable for future expansion. • Leverages existing LIS investments. 12 • • • 5.2. Does not require regional EMPI for implementation. Once implemented, requires significant resources and expertise to maintain (i.e. dynamic nature of Laboratory Medicine requires constant changes to test menus, instrumentation, reference intervals, reporting limits etc.). Both fee-for-service and shared services models of reimbursement may be appropriate. Factors to consider include the level of variation among system usage and whether the sharing of lab services is sufficient to warrant financial integration. OLIS & LIS Integration The OLIS project is a province-wide repository of laboratory data. On its own, it is not a replacement for regional LIS integration and connectivity. However, OLIS represents both a requirement and an opportunity in laboratory services planning. LIS integration initiatives must consider how their data will integrate with OLIS when it connects with the organizations involved. At the same time, planners may consider how to leverage the connectivity that OLIS provides. This study found one example of an initiative that proposed to use the connectivity of OLIS to act as an interface gate between laboratory partners. In all cases, there are two main issues which much be considered when determining how a regional LIS network will fit with OLIS. These are interfacing and nomenclature. 5.2.1. Interfacing OLIS has provided interface specifications developed based on the HL7 2.3.1 Standard, which will form the basis for any data interactions between OLIS and laboratory service providers. OLIS also plans to support a chosen version of HL7 (between 2.4 and 2.7). Version 3.0 will be supported when it is sufficiently defined and accepted in the laboratory domain by the HL7 standards organization. OLIS supports flexible deployment options. In an integrated regional LIS model, it can be deployed through a single connection point shared by each stakeholder in the regional environment, or may be deployed using separate connections at each location. Each region may choose a deployment option that meets its needs. 5.2.2. Nomenclature OLIS has defined standard nomenclature for orders and results. These standard nomenclatures can be deployed for use within organizations or can be mapped to local nomenclatures. To facilitate the introduction of standard nomenclatures, it is recommended that stakeholders review and understand the OLIS nomenclatures. OLIS provides a mapping tool to facilitate mapping, if this option is selected. 5.3. LHINs & LIS Integration As a system of governance that integrates multiple health care sectors in a region, there are parallels between the LHIN IT strategy and regional laboratory data sharing strategies. Although LHINs may not directly affect the strategic decisions of regional sharing initiatives, any planning needs to consider how an initiative may affect LHIN IT planning and whether the involvement of 13 LHIN governance may be leveraged to benefit the formation and funding of a LIS integration model. 5.3.1. Governance To date, LIS integration initiatives have been governed and managed through existing regional structures or specific inter-institutional agreements. However, as LHIN implementation proceeds and LIS connectivity and integration expands, it is anticipated that the governance and management of LIS integration networks will transition and evolve into the LHIN governance structures. There are three distinct scenarios of LIS connectivity which must be accommodated within future LHIN governance structures: i) Intra-LHIN LIS connectivity involving the integration of two or more hospital LISs within the same LHIN ii) Inter-LHIN LIS connectivity involving two or more hospital LISs across two or more LHINs iii) Connectivity of integrated LIS networks with provincial systems such as OLIS, PIMS and eCHN For each of these scenarios, a LIS integration management and governance structure that supports and compliments the laboratory medicine and institutional structures, will need to be developed. Governance may consist of simple service level agreements (SLA) that spell out the required levels of performance and the consequences of failure or a full memoranda of understanding (MoU) that provides a detailed understanding of ownership, control, and protection for partners. 5.3.2. Finance To date, most LIS integration initiatives have employed one of two financial models: costs are either apportioned out by usage of the system or by the relative size of the organizations involved (e.g. total budget or bed size). Regardless of the model, LHIN boundaries should not affect the financial governance of these projects. The key consideration is that existing partnerships not impede LHIN integration and planning and vice versa. 14 6. CRITICAL SUCCESS FACTORS Throughout course of this research, contacts for each initiative were asked to highlight the issues encountered in the process of implementing regional LIS integration and connectivity solutions that had the most impact on the success of the project. Although all of the initiatives approached their solutions in different ways, many commonalities were observed in these critical success factors. The most prominent included: • Strong leadership from top of corporate structure • Joint vision • Sufficient funding • Laboratory medical/scientific and technical staff support • Rigorous project management • Standardization • Vendor participation • Sufficient resources for ongoing operation and maintenance 7. BENEFITS The importance of ensuring that the benefits of regional initiatives were seen by all partners and communicated to organizations and their staff was emphasized by many. The benefits of regional lab data sharing most commonly illustrated included: • Timely information to those that need it • Faster turnaround times • Reduction of transcription errors • Cost sharing to help smaller institutions take advantage of economies of scale • Improved data quality • Enhanced data mining possibilities • Future projects better able to build upon success (key enabler for future consolidation) 15 8. ACKNOWLEDGEMENTS The OHA and its Ontario Hospital e-Health Council would like to recognize the important contribution of the HLISAG in the development of this report. The OHA and Hospital e-Health Council would also like to thank those contributors who generously volunteered their time and efforts by completing an Environmental Scan and reviewing other materials, such as profiles and diagrams. A special thanks to Lewis Hooper, CIO Central East LHIN, for sharing his insights on the LHINs in relation to lab data sharing. In addition, many thanks to the OLIS project and staff, and SSHA for their support and assistance. Hospital Laboratory Information Systems Advisory Group (HLISAG) Members: Dr. Sherry Perkins (Chair) The Ottawa Hospital Lindsay Campbell Ontario Laboratory Information System (OLIS) Project Lan Djang Ontario Hospital Association Derek Graham Temiskaming Hospital Dr. Tadaaki Hiruk Ontario Association of Pathologists Bev Junnila Thunder Bay Regional Health Sciences Centre Murray Kaufman Sunnybrook Health Sciences Centre Susan Kewin Smart Systems for Health Agency Dr. Robert Lannigan London Health Sciences Centre Susan Leask Lakeridge Health Corporation Cheryl MacInnes Cambridge Memorial Hospital Martha Murray Ontario Hospital Association Christine Probst Hamilton Regional Laboratory Medicine Program Bonnie Reib The Hospital for Sick Children Melissa Tamblyn/Pat Mason Cancer Care Ontario Cathie Trayner Kingston General Hospital 16 9. APPENDIX A: ANALYTICAL SUMMARY OF ENVIRONMENTAL SCANS 1. Sum Total of the Survey • 17 total: • 2 single organization profiles (William Osler Health Centre [WOHC], Trillium Health Centre [Trillium]) • 2 single organization profiles that include regional sharing of lab services (Sunnybrook Health Sciences Centre [SHSC], Mt. Sinai Hospital [MSH]) • 11 regional sharing initiatives • 2 province-wide initiatives (Pathology Information Management System [PIMS], electronic Child Health Network [eCHN]) 2. Which ones had current interchange and which ones were future plans? • 13 profiles were of current regional data sharing • 4 profiles described planned future regional data sharing, with only limited data sharing in current context 3. Types of solutions • Multi-LIS Vendor, Multi-System (completely separate systems and LIS databases, with distributed control, but interfaced to each other) • 9 total (most regional sharing initiatives fall under this category) • Single-LIS Vendor, Multi-System (Single vendor with multiple LIS platforms integrated/connected through an interface engine) • 2 profiles exclusively fell under this category, while the single vendor/multiple system elements were found in one other profile that fell under the multivendor/multi-system category overall • Single-LIS, Single-System (complete integration of lab systems) • 4 total (including 2 region-wide sharing initiatives) • Province-wide Data Repository (unidirectional data feed, with a bidirectional viewer functionality) • 2 total (PIMS, eCHN) 4. Governance • Cooperative governance was not applicable to single organization profiles or provincewide profiles (though some form of cooperative decision making does take place at the operational level) • Most common strategic governance involved a cooperative structure, with a single board or steering committee oversight (8 out of 10 regional sharing initiatives) • Exceptions were Toronto Medical Laboratories (TML), which, while a joint partnership exists, is governed as a separate service provider with clients purchasing services; and East 2 (E2), where each organization governs their own lab services 17 5. Funding • Capital costs may be funded under different models from operational costs • Cost sharing plan under cooperative governance used in 3 models (Hamilton Regional Laboratory Medicine Program [HRLMP], Northwest Health Network [NWHN], London Laboratory Services Group [LLSG]) • Fee for service used in 6 models (SHSC, MSH, TML, Central West Microbiology [CW Micro], E2, Inter-Hospital Laboratory Partnership[IHLP]) • 2 examples of regional level governance with a single combined budget (North Eastern Ontario Network [NEON] and Eastern Ontario Regional Laboratory Association[EORLA]) 6. Primary LIS Vendors • Listing of primary LIS vendors involved in each profile Profile Hamilton Regional Laboratory Medicine Program (HRLMP) Sunnybrook Health Sciences Centre (SHSC) Mt. Sinai Hospital (MSH) Toronto Medical Laboratories (TML) Central West Microbiology (CW Micro) East 2 (E2) Central East 1 (CE1) Eastern Ontario Regional Laboratory Association (EORLA) Pathology Information Management System (PIMS) electronic Child Health Network (eCHN) Inter-Hospital Laboratory Partnership(IHLP) Northwest Health Network(NWHN) North Eastern Ontario Network (NEON) London Laboratory Services Group (LLSG) William Osler Health Centre (WOHC) Grey Bruce Health Services (GBHS) Trillium Health Centre (Trillium) Primary Vendor(s) Meditech, HBO McKesson MISYS (General Lab & CoPath), Mediware (Blood Bank) SCC GE Healthcare, Cerner (CoPath), SCC, Mediware Meditech, HBO, McKesson Meditech, MISYS Meditech, GE Healthcare (Triple G) Cerner, Lab Vision Proprietary Proprietary Meditech Meditech Meditech Cerner Meditech Cerner Meditech Notable: 10 involved Meditech, 5 involved Cerner 7. HL7 version • Version 2.3 was most commonly used • Only NWHN (v.2.2) did not support v.2.3 • Most common versions were 2.1, 2.2, and 2.3, largely dependent on the systems involved in the integration projects • Only PIMS reported support for higher versions of HL7 (up to v.4.9) 8. Network Connectivity • SSHA was the primary network connectivity provider for 11 profiles, and was also used for connectivity with certain TML clients • The other provider noted was Bell 18 9. Key Common Critical Success Factors • Strong leadership from top of corporate structure • Joint vision • Sufficient funding • Physician and staff support • Rigorous project management • Standardization • Vendor participation • Sufficient resources for ongoing operation and maintenance 10. Key common Benefits • Timely information to those that need it • Faster turnaround times • Reduced transcription errors • Cost sharing helps smaller institutions take advantage of economies of scale • Improved data quality • Enhances data mining possibilities • Future projects better able to build upon success (key enabler for future consolidation) 19 10. APPENDIX B: FINAL ENVIRONMENTAL SCAN QUESTIONNAIRE Guidelines for Investigation PURPOSE • • To understand the different models currently employed, or to be implemented, in the field for lab data exchange between hospitals and other external institutions and providers. To understand the purpose, transaction model details, data shared, stakeholders involved, and best practice outcomes from each existing model. SCOPE Transactions To examine the full spectrum of business processes included in a lab information exchange transaction. This includes: • Uni-directional exchange (e.g. Health care Provider/Lab / LIS data repository) • Bi-directional exchange (e.g. Health care Provider/Lab ' Lab/Health care Provider; Orders and Results) • Requisite peripheral information and materials that must move with lab data (e.g.: specimens/patients) Participants • The focus of the scan was on acute care hospitals as stand-alone institutions or as members of regional networks/partnerships. • Selection criteria for participation in the survey centered on hospitals/hospital groups who were currently, or had plans to, electronically exchange laboratory data between laboratory providers (e.g. other hospitals, private laboratories, public health laboratory). • Hospitals that only exchange laboratory data internally with clinical repositories or “niche” clinical systems were not included. METHODOLOGY • Identify target respondents based on existing lab information exchange projects and network via HLISAG membership. • Informal one-on-one discussions with representatives from each lab info exchange project to be carried out by identified HLISAG members. • Following semi-structured interview guide to obtain qualitative and quantitative description of model to include process flows of both data and specimen/patient exchanges. DRAFT INTERVIEW GUIDE 1) “Screener Question”: Do you currently share laboratory data electronically (either unidirectionally or bidirectionally) with other laboratory service providers and other health care providers who exchange lab data on a regular basis (e.g. CCO, external QA/QC monitoring, other institutions and individual providers)? If not, proceed to Question 7 (which asks about future plans). 20 2) A) What type of regional information exchange are you participating in? • Repositories and clinical “niche” systems (unidirectional) • Orders & Results (bi-directional) • Laboratory to Administrative systems (e.g. Finance/General Ledger, ADT, HIS, Pharmacy etc). B) If “yes” to any part of A, does your information exchange model support movement of specimens, patients or ancillary information beyond the message exchange (e.g. Information beyond the order/results, like image data, Pedigree file extracts, etc.)? 3) Information Exchange Model: Probes to include: • Who is involved? (names of institutions) • Governance model • Did you consider more than one model? If yes, why did you choose the one you are currently using? • Do you use more than one model? If yes, why? 4) How is the system administratively controlled (i.e.: planning and governance)? 5) How is the system funded (one-time start up costs vs. ongoing operational costs)? 6) Describe the lab data exchange model. (This section will provide the most detail for the environmental scan) Please describe the following: • LIS (vendors, application software products and current versions, installation configuration, interfaces to other systems) • Measures that have been taken to support accurate patient identification and matching • Process Model: Flow of orders, specimens, patient movement and results. (obtain flow chart if possible; see attached diagram) • Relationship to all the systems involved (clinical, pharmaceutical, ADT, administrative) (obtain diagram if possible) • How quality assurance is addressed: • Testing of data transmission prior to go live ¾ Is there a test plan to validate information sharing prior to implementation? ¾ Is there a procedure to document and approve the validations of information sharing prior to implementation? • Integrity of data transmission (data integrity) ¾ Are the formats and methods for transmitting shared information standardized? ¾ Are data connections periodically checked for functionality, and how? • Correctness of data transmitted (data validation) ¾ What mechanism is in place to verify that shared information is accurately transmitted? 21 ¾ • • Are mapping tables that are required for information sharing periodically verified in all locations, and how? • Tools and processes necessary to ensure quality data ¾ What backup procedures are in place to ensure that loss of shared information is prevented? ¾ What mechanism is in place to report malfunctions in information sharing? ¾ What mapping tools and processes are in place to enable automated (or partially automated) table updates of items such as nomenclatures when a new release becomes available? ¾ What processes are in place to add a new code or value required for reporting against to a coding scheme on a temporary basis? (i.e. before a scheduled release or update?) • Process for correcting data quality issues ¾ What support mechanism is in place to address malfunctions in information sharing? Standards employed to support the exchange, and future plans to implement standards: • Physical (connectivity, security, audit) • Data types (discrete data, report image, structured report) • Messaging (e.g. HL7 and what version, other) • Nomenclature/Coding (LOINC, ICD-10, SNOMED, other) How is security/confidentiality of patient information and access to the LIS addressed? • Is the SSHA Pipeline used at your organization for data transfer of laboratory results? Yes/No • Does your organization have a firewall? Yes/No • Do you have controlled access for internal staff to the LIS utilizing User ID and Passwords? Yes/No • Do you have a system in place to control and monitor the external access of laboratory data by health care professionals? Yes/No • Do you have a quality assurance program to monitor the access to laboratory results for clinical use only? Yes/No • If yes, how often do you monitor this access? Weekly/Monthly/Annually/Other please specify________________ • Lab accreditation status (OLA, CAP, ISO, other) • Pre/Post operational & clinical benefits published? 7) If you have a plan for regional interaction between now and the next 24 months, please tell us about it: 1. Describe the planned exchange model as per the questions above, in as much detail as is currently available. 2. Indicate the planned timing of this project. 3. How will this integration be funded? a) One-time costs for building integration b) Ongoing operations and support 4. For existing interface engines, how will change be managed as associated systems are upgraded? 22 8) Please describe how your lab data “information exchanges” have benefited your institution. 9) What have been some of the critical success factors/hurdles that were overcome for successful implementation? 23 11. APPENDIX C: FINAL ENVIRONMENTAL SCAN PROFILES 11.1. Central East 1 Central East 1 (CE1) Laboratory Sharing Initiative As of August 2006 Lab Reform Region Central East Description Proposed interface of orders and results between CE1 hospital LISs and main reference lab provider (MDS). Category Multiple LIS Vendor/Multiple System Integration Extent of Sharing Electronic sharing of orders and results between each CE1 hospital and MDS. Status/Timeframe Proposed project. Planned to take place over next 12 - 24 months. Organizations Involved 7 CE1 hospital sites. See attached list below. MDS Governance Model Initiative will be governed as part of Lab IS functions at each member hospital. Funding TBD Assume start-up costs and operating costs will be separately funded by each hospital. Data Sharing Model Model 1: Individual hospital LISs will connect point-to-point with MDS. Model 2: Individual hospital LISs will connect to MDS via hub and spoke interface using OLIS. Link to Diagram Model 1 Model 2 LIS’s used Individual hospitals have their own LISs, which are either Meditech or Triple G. MDS has a proprietary system. HL7 Version 2.3 Network Connectivity CE1 will use SSHA pipelines provided to each hospital and MDS for connectivity. Nomenclature TBD QA Data/ Patient Identifiers TBD Connectivity TBD Nomenclature/Mapping TBD Corrective Actions TBD Comments on Privacy & Security User access will be based on each individual site’s policies. Daily auditing occurs based on random sampling. 24 11.1. Central East 1 Central East 1 (CE1) Laboratory Sharing Initiative As of August 2006 How SSHA Fits in the Picture SSHA will be the primary network infrastructure provider. How OLIS Fits in the Picture OLIS will be interfaced to the hospitals and (in Model 2) would provide the interfacing and routing between hospital sites and MDS. Critical Success Factors Capital funding for IT projects. Sufficient resources for ongoing operation and maintenance of data sharing. Benefits of This Initiative Eliminating manual transcription of results into the LIS. Eliminate transcription errors. Provide real-time results thus improving turnaround time. Save operating dollars, through the reduction in paper purchased. Reassign MLT hours to other tasks within the laboratory. Key enabler for further regional consolidation. Contact Info Susan Leask Program Manager Lakeridge Health Corporation sleask@lakeridgehealth.on.ca 905-576-8711 x3464 Participating Central East 1 Hospitals • Campbellford Memorial Hospital • Lakeridge Health – Oshawa • Lakeridge Health – Bowmanville • Lakeridge Health – Port Perry • Northumberland Hills Health Centre • Peterborough Regional Health Centre • Ross Memorial Hospital 25 CE1 Model 1 (Point to Point) Clinician Electronic connection Clinician Manual/physical connection Client Site 1 (LIS 1) Client Site 2 (LIS 2) HL7 Interface HL7 Interface Orders/Results Specimens Specimens OLIS HL7 Interface Orders/Results MDS (Proprietary LIS) 26 Same model is used for all sites CE1 Model 2 (Hub and Spoke) Electronic connection Clinician Clinician Client Site 1 (LIS 1) Client Site 2 (LIS 2) HL7 Interface Manual/physical connection HL7 Interface Specimens Specimens Orders/Results MDS (Proprietary LIS) 27 Same model is used for all sites 11.2. Central West Central West Region – West Node Microbiology (CW Micro.) As of August 2006 Lab Reform Region Central West Description Regional consolidation of microbiology lab services at Grand River Hospital (GRH). Category Multiple LIS Vendor/Multiple System Integration Extent of Sharing Full sharing of microbiology orders/results and specimens to and from a regional referral lab. Status/Timeframe Implemented project. Plan to extend to more hospitals in the region and find more regional opportunities in other lab divisions. As of late 2005, Groves Memorial Hospital (GMH) to be integrated and planning for further regional opportunities is ongoing. Organizations Involved GRH, St. Mary’s General Hospital (SMGH), Cambridge Memorial Hospital (CMH) Governance Model Contract fee-for-service arrangement between separate organizations. GRH is responsible for the microbiology service. Each partner organization is responsible for their own interface to the system. Funding Contract fee-for-service. The system has been funded by each hospital’s global budget. Data Sharing Model Microbiology orders and results are interfaced between client hospital’s LIS and referral lab’s LIS, with specimens transported to referral lab and re-bar coded at lab. Link to Diagram Click here for SMGH-GRH process diagram. LIS’s used Meditech 4.9, McKesson Horizon Laboratory System HL7 Version 2.3 Network Connectivity SSHA network is used for transmitting orders and results. Nomenclature ICD 10 QA Data/Patient Identifiers Faxed results are confirmed at receiving site for the first few weeks. Connectivity All interface errors are printed and reviewed. Nomenclature/Mapping There is a defined customer managed process of change control, unit and integrated testing which allows for tabular values to be changed in test, then production as needed. Corrective Actions There are standardized procedures for reporting any issues requiring attention via a 24/7 helpdesk. Comments on Privacy & Security The system has audit capabilities and there are random audits of routine processes and VIP audits performed routinely by the Information Services Department. 28 11.2. Central West Central West Region – West Node Microbiology (CW Micro.) As of August 2006 How SSHA Fits in the Picture Network connectivity provider How OLIS Fits in the Picture TBD Critical Success Factors Technology: development of the bidirectional Meditech/McKesson interface for the microbiology regionalization. Change management. Quality business cases. Organizational buy-in. Benefits of This Initiative Creation of a platform to facilitate the building of further successful laboratory reform plans. Increased regionalization opportunities: The Waterloo Wellington Laboratory Services Strategic Planning Team is currently working on several initiatives to be reported on in September 2006. Contact Info Cheryl MacInnes Director DI & Health Care Support Services Cambridge Memorial Hospital cmacinnes@cmh.org 519-621-2330 x2277 Process Diagram (SMGH-GRH Interface) Electronic connection Manual/physical connection HL7 interface Clinician Orders/results Client Hospital (Client LIS) Grand River Hospital (McKesson LIS) Client barcode McKesson barcode Specimens 29 Centralized Micro. Lab Same model is used for all client sites 11.3. East 2 East 2 (E2) As of October 2006 Lab Reform Region East 2 Description Kingston General Hospital (KGH) is the reference laboratory for most testing in the region. The Outreach business unit of KGH receives specimens from clients and facilitates the reporting and billing. At present, there is no standardized electronic connectivity in the region. Category Multiple LIS Vendor/Multiple System Integration Extent of Sharing Full sharing of results for referred pathology and esoteric tests through a mixture of non-standardized electronic connection and delivery of hard copy. Status/Timeframe Integration discussion ongoing. Organizations Involved Kingston General Hospital (KGH), Brockville General Hospital, Quinte Healthcare Corporation, Lennox and Addington County General Hospital, Hotel Dieu Hospital (HDH), Perth and Smith Falls District Hospital, Weneebayko General Hospital, Providence Continuing Care Centre (PCCC), St. Mary of the Lake Hospital, Kingston Psychiatric Hospital, Canadian Forces Base. Hospitals In-Common Laboratory (HICL) Governance Model Each hospital corporation operates independently. Kingston General Hospital maintains a Rapid Response Lab at Hotel Dieu Hospital. Funding Individual global budgets; KGH bills HICL and client hospitals. Data Sharing Model All orders are on paper, results are sent back to clients via difference modes (i.e. electronic download to client HIS, HL7 interface, remote printing or paper reports via courier). Link to Diagram Click here for process diagram. LIS’s used Meditech, Misys, Rubicon (HICL proprietary), Health Vision (being phased out). HL7 Version 2.x, depending on system Network Connectivity SSHA provides network connectivity between all the hospitals Nomenclature LIS dependent. SNOMED-CT for pathology at KGH. KGH will incorporate OLIS nomenclature standards with new LIS. QA Data/Patient Identifiers Reference samples sent to KGH are entered into either the LIS or Patient Care system of KGH Connectivity KGH is connected to Quinte Health Corporation via VPN, to Perth and Smiths Falls via dial-in Modem, and to Lennox and Addington and HICL via HL7 interface. Lennox and Addington uses HICL proprietary system. Nomenclature/Mapping n/a 30 11.3. East 2 East 2 (E2) As of October 2006 Corrective Actions Regional partners notify KGH, and Outreach customer service staff addresses the problem. Comments on Privacy & Security Courier or print on demand for hard copy reports. Electronic access is by username and password. How SSHA Fits in the Picture Network connectivity provider. Limited functionality enabled to date. How OLIS Fits in the Picture TBD Critical Success Factors Persistent cooperation and collaboration within the region Strong leadership and planning Vision of integration identified in E2 Lab Alliances Strategic plan Benefits of This Initiative Improved access to patient lab information across the region Reduced duplication of work Improved turnaround times for referred tests Contact Info Norma Layno Kingston General Hospital laynon@kgh.kari.net 613-533-2828 Cathie Trayner Kingston General Hospital traynerc@kgh.kari.net 613-549-6666 x3662 31 E2 Process Diagram Perth & Smiths Falls District (Meditech) Brockville General (MISYS) Pathology Reports (secured fax line) Paper Results Quinte Healthcare (Meditech) Weneebayko General Canadian Forces Base Lennox & Addington County General (Rubicon, shared w. HICL) Remote Printing Paper Results Paper Results Paper Results Unidirectional Download via Meditech Specimens, Paper Orders HL7 Interface KGH-HDH Outreach Service (Lab Vision) Results Results Electronic connection Results Manual/physical connection HL7 Interface HICL (Rubicon proprietary) 32 Specimens, Paper Orders 11.4. Eastern Ontario Regional Laboratory Association Lab Reform Region Eastern Ontario Regional Laboratory Association (EORLA) As of July 2006 East 1 Description Planned full regional integration and connectivity of laboratory services between all hospital partners in EORLA. Category Multiple LIS Vendor/Multiple System Integration Extent of Sharing When fully operational, will include sharing of orders/results, regional integration of laboratory services and consolidation to a single funding envelope for hospital based laboratory services. Status/Timeframe On-going project. Long history of regional laboratory collaboration. Currently significant amount of specimen referral within region, however, majority of interinstitution transmission of orders and results is paper based. LIS integration planning completed (model and connectivity estimates confirmed through 3rd party review in early 2006). Implementation initiated in Spring of 2006 through RFP process to select project management/implementation consultants. Implementation vendor to be selected early fall 2006, connectivity to be built and phased in over 12-18 months with regional data repository and OLIS connectivity to follow. Organizations Involved 16 member hospitals. See attached list. Pathology reports are interfaced to CCO (not depicted here). Governance Model Non-profit shared services corporation governed by a board representing member hospitals. Funding Some funding received through MOH Back Office Transformation (“Ontario Buys”) initiative to support start-up costs. Once fully implemented on-going operations will be funded by global EORLA budget that combines lab budgets of member hospitals. Data Sharing Model All individual hospital LISs are connected to a regional data hub via HL7 messaging, with orders transmitted and results viewed by the individual LISs at each site. Link to Diagram Click here for process diagram of the planned future state, with regional data repository and OLIS connectivity. LIS’s used Each site runs its own LIS. Two regional referral labs use Cerner Classic 3.06 (TOH) and LabVision (CHEO). Other supported LISs include MDS’ proprietary LIS, Triple G, Sysware, Anzer, Medisolution and Meditech (implementation to be completed Sep. 2006). HL7 Version 2.1, 2.2, 2.3 (depending on site) Network Connectivity EORLA uses SSHA pipelines provided to each hospital for connectivity. 33 11.4. Eastern Ontario Regional Laboratory Association Nomenclature QA Eastern Ontario Regional Laboratory Association (EORLA) As of July 2006 EORLA is establishing its own nomenclature with plans to align closely with OLIS. Data/Patient Identifiers TBD Connectivity TBD Nomenclature/Mapping TBD Corrective Actions TBD Comments on Privacy & Security User access will be based on each individual site’s policies. Daily auditing occurs based on random sampling. PHIPA. How SSHA Fits in the Picture SSHA is the primary network infrastructure provider. How OLIS Fits in the Picture The next phase of the project will enhance the regional data hub with a regional data repository (OACIS). It has yet to be determined whether this repository will then feed orders and results to OLIS or if individual sites will feed orders and results directly from their individual LIS. Critical Success Factors Capital funding for IT projects. Agreement on regional governance. Sufficient resources for ongoing operation and maintenance of data sharing, especially of the regional data hub. Benefits of This Initiative Decreased turnaround time in terms of access to results. Reduction of data entry errors and erroneous test requests. Key enabler for further regional consolidation. Contact Info Dr. Sherry Perkins Head - Division of Biochemistry The Ottawa Hospital slperkins@ottawahospital.on.ca 613-798-5555 x16107 Participating EORLA Member Hospitals • Almonte • Kemptville • Arnprior • Montfort • Carleton Place • Pembroke • Cornwall Community Hospital • Queensway Carlton Hospital (QCH) • Children’s Hospital of Eastern Ontario (CHEO) • Renfrew • St. Francis • Deep River • The Ottawa Hospital (TOH) • Glengarry • Winchester • Hawkesbury 34 EORLA Process Diagram (Planned Connectivity) Electronic connection Manual/physical connection Clinician orders Specimens EORLA Site Lab (Site Lab LIS) Results viewing Specimens orders/site lab results HL7 Interface Regional LIS Integration HUB HL7 Interface orders/results CHEO Regional Lab (Lab Vision LIS) OLIS* TOH Regional Lab (Cerner LIS) * Connectivity to EORLA system TBD 35 Histology Slides Lab Data Specimens Regional OACIS Data Repository Same model is used for all 16 client sites 11.5. electronic Child Health Network electronic Child Health Network – Health Information Network application (eCHN – HiNet) As of September 2005 Lab Reform Region All Description eCHN currently shares ADT, Laboratory, Diagnostic Imaging and Health Records data with 55 member sites via a centralized data repository (as of September 2005). Hospital for Sick Children (HSC) connection with eCHN is depicted. Category Province-wide Data Repository Extent of Sharing eCHN HiNet includes all clinical data (Laboratory, DI, eMAR- electronic Medication Administration Report, as well as electronic reports including scanned documents, and ADT information) that the member hospital is willing to share electronically. Status/Timeframe Implemented project. HSC is working with eCHN to create a direct interface with MDS. This will allow the direct transfer of referred lab results from MDS to the HSC LIS and to eCHN HiNet. Anticipated by Q3 2006. Organizations Involved 55 member child healthcare provider sites and HiNET users in the community. Click here for a list. Governance Model Formally, as a non-profit organization, eCHN must report to, and remain accountable to, the Ministry of Health and Long-Term Care of Ontario (MOHLTC). eCHN manages the data interchange. There is a formal process requiring approval from both the member organization and eCHN prior to implementing any initiatives involving both organizations. Funding eCHN is fully funded (both implementation of new member sites and ongoing operating costs) by the MOHLTC. Data Sharing Model Unidirectional feed of laboratory data from member sites into a centralized eCHN repository. HiNET users can then view the data. Link to Diagram Click here for connectivity model. LIS’s used eCHN is vendor neutral and accepts disparate Lab data in HL7 format from all LIS vendors, including MISYS, Lab Vision, Meditech, SoftLab, Sunquest, Triple G. HL7 Version 2.2 Network Connectivity SSHA connectivity between eCHN member sites, VPN access also supported for doctor offices. Nomenclature eCHN has normalized all member site clinical data to the LOINC and SNOMED standard. 36 11.5. electronic Child Health Network electronic Child Health Network – Health Information Network application (eCHN – HiNet) As of September 2005 QA Data/Patient Identifiers All data is thoroughly tested prior to implementation and retested during every software upgrade. Quality analysis is also preformed during sample checking. Connectivity eCHN checks all data using their suite of ECR (Error Correction and Recovery) tools and process for day to day verification. Nomenclature/Mapping Lab data dictionary extracts are “synchronized” (i.e. checked for changes) against eCHN-MED on a daily basis (error automatically flagged at part of ECR tool suite and data dictionary manually evaluated whenever upgrade takes place. For example, this process was used during HSC’s Softlab to Sunquest upgrade. Corrective Actions eCHN has a 24/7 help desk and works with the member sites to address and correct any malfunctions or data quality issues. There is a formal Error, Correction, and Recovery (ECR) process in place at eCHN to address and resolve any malfunctions in information sharing between member sites. Any issues that are discovered are addressed immediately by the eCHN Data team subject matter experts. The data quality issues are managed via the ECR tools, which include a mechanism to exchange/review information with the sites. Comments on Privacy & Security Security- user access and password controlled, 3 factor authentication is currently being implemented. Audit feature built into application for the user level, on the network side – firewall audit logs are reviewed both manually and by automated tools. How SSHA Fits in the Picture SSHA provides connectivity between eCHN member sites. How OLIS Fits in the Picture TBD Critical Success Factors Through funding from the MOHLTC, HSC and eCHN were able to work together and provide the first successful electronic health record in Ontario. Through the process of sharing data, HSC was able to gain access to industry subject matter experts and therefore improve their own internal processes. eCHN’s normalization and QA work gives HSC an extra level of assurance and integrity. Benefits of This Initiative The eCHN initiative supports the sharing of paediatric data with the healthcare provider community. Member sites, such as HSC, can therefore provide timely and accurate information on all patients to their direct caregivers without those caregivers being physically present. Contact Info Bonnie Reib Managing Director, Department of Paediatric Laboratory Medicine The Hospital for Sick Children bonnie.reib@sickkids.ca 416-813-8958 37 Participating eCHN Sites • Bloorview Kids Rehab • Smooth Rock Falls Hospital • Chapleau Health Services • Sudbury Regional Hospital • Children's Hospital of Eastern Ontario • The Hospital for Sick Children • Cornwall Community Hospital • Temiskaming Hospital • Grandview Children's Centre • Thessalon Hospital • Englehart and District Hospital • Thunder Bay Regional Hospital • Kirkland and District Hospital • Timmins and District Hospital • Lake of the Woods Hospital • West Nipissing General • Mattawa General • William Osler Health Centre • North Bay General Hospital • Northeast Mental Health Care Orillia Soldiers' Memorial Hospital • Algoma CCAC • CCAC of the District of Thunder Bay • Rouge Valley Health System (Ajax & • CCAC of Timiskaming • Pickering) • Cochrane District CCAC • St. Joseph's Health Centre (Toronto) • Manitoulin-Sudbury CCAC • St. Elizabeth Health Care • Muskoka East Parry Sound CCAC • Sault Area Hospital • Near North CCAC • Sensenbrenner Hospital • Ottawa CCAC • West Parry Sound CCAC 38 eCHN Process Model eCHN-HiNet Client Combined Data Clinician Electronic connection Manual/physical connection Orders/results Specimens Site ADT System Site Lab (Site Lab LIS) Orders/results Combined Data Patient data Client Site – eCHN Interface Engine Combined Data eCHN-HiNet 39 Model representative of connectivity with most sites 11.6. Grey Bruce Health Services Grey Bruce Health Services (GBHS) As of August 2006 Lab Reform Region Southwest Description Installation of a single Laboratory database at GBHS-Owen Sound to serve eleven hospital laboratories and one emergency aid clinic within Grey and Bruce Counties. Established links to at least one physician clinic. Category Single Vendor/Multiple LIS integration Extent of Sharing Grey Bruce Health Network consists of eleven hospitals and one emergency aid clinic (a Cluster group within the Southwest region). Status/Timeframe System testing is currently underway with a projected roll out date for all sites of August 2006. Organizations Involved Hospitals include GBHS-Owen Sound, GBHS-Meaford, GBHS-Markdale, GBHS-Wiarton, GBHS-Lions Head, GBHS-Tobermory Clinic, GBHSSouthampton, SBGHC-Kincardine, SBGHC-Walkerton, SBGHC-Durham, SBGHC-Chesley. Although part of the IHLP Cluster group, Hanover Hospital is also a member of the Grey Bruce Network and is included in this project. Governance Model The relationship is on a contractual basis between GBHS and the other members of the Grey Bruce Health Network. Services are provided to the other members by GBHS and governance decisions are made by GBHS. Funding Capital costs have been borne by GBHS and portions thereof supported by user facilities based on volume. Data Sharing Model All facilities are linked directly to a single Cerner database at the GBHS Owen Sound site using Citrix software. All of the rural sites are connected to a central server/database in Owen Sound via Citrix. Citrix use is necessary for all LIS transactions for the rural sites. Link to Diagram Click here for process diagram. LIS’s used All sites will be running Cerner PathNet LIS and linked directly to the Cerner HIS system currently in place. HL7 Version HL7 version 2.3 Network Connectivity Citrix software using encrypted microwave transmissions will provide the system links to the various sites. The capability of using the SSHA pipeline is also available. Connectivity should switch to fibre optic cable in the very near future. Nomenclature Most of the nomenclature used on the system was standardized from previous systems. The Cerner system allows for both Primary and Ancillary synonyms so any discrepancies can be handled in that fashion. This will also allow for easy use of OLIS nomenclature once it is in widespread use. QA Data/Patient Identifiers Institution Medical Record numbers, OHIP numbers, Last Name, First Name and DOB are all used to ensure integrity of data. Connectivity Monitored on a continuous basis by the IT Dept. Periodic checks of data integrity. Nomenclature/Mapping Verified as changes require. 40 11.6. Grey Bruce Health Services Corrective Actions Grey Bruce Health Services (GBHS) As of August 2006 Reported errors are logged both in a local access database and the Cerner Service Request logs. All instances are handled in a timely fashion. Comments on Privacy & Security Secure network connections, firewalls and encryption combine to ensure confidentiality of information. How SSHA Fits in the Picture TBD How OLIS Fits in the Picture TBD Critical Success Factors Standardization of data and equipment across all sites has minimized the amount of build and maintenance required on the system. We see a large portion of the patient population moving between sites so physicians at all sites will now see a standard report format with similar ranges for similar machines. Use of the Cerner Accelerated Solutions team has dramatically reduced the timeline required for a project of this magnitude. Executive support from all three Corporations involved has allowed the secondment of dedicated personnel to maintain timelines on the project. The use of senior, knowledgeable Technologists on the team has made decision making and data gathering much easier than might otherwise have been the case. Benefits of This Initiative Use of a single database and shared laboratory information between all the Grey-Bruce sites. Standardized nomenclature and reference ranges across all sites. Standardized chart reports for all physicians in the area. A complete specimen tracking system for all samples processed at the various sites, exchanged between sites or referred to outside laboratories. Opportunity for extensive data mining using a single database. Opportunity to move to a paperless reporting system. Contact Info Dave Ford Corporate Lab Manager Grey Bruce Health Services dford@gbhs.on.ca 519-376-2121 ext. 2135 41 GBHS Process Diagram Electronic connection Manual/physical connection GBHS Referral Site Lab (Cerner PathNet LIS) Citrix GBHS Owen Sound Site Cerner DB Specimens Orders/Results Citrix Orders/Results GBHS Client Site (Cerner PathNet LIS) Clinician 42 Same model is used for all sites 11.7. Hamilton Regional Laboratory Medicine Program Lab Reform Region Hamilton Regional Laboratory Medicine Program (HRLMP) As of August 2006 Central South Description Lab service amalgamation between two hospital organizations Category Single/Multiple LIS Vendor/Multiple System Integration Extent of Sharing Lab services are rationalized at different sites depending on clinical requirements and specialties. In addition, referred orders/results and specimens are shared within the lab system. Status/Timeframe Administratively, integration is complete. From an LIS point of view, consolidation to one LIS is planned for 2007. Organizations Involved Hamilton Health Sciences (HHS) and St. Joseph’s Healthcare (SJHC), Hamilton. West Lincoln Memorial Hospital, HICL and Canadian Medical Laboratories (in testing) are client sites. CCO (not depicted). Governance Model The Hamilton Regional Laboratory Medicine Program (HRLMP) is a jointly owned and operated clinical service program of HHS and SJHC corporations, and is affiliated with the McMaster University Faculty of Health Sciences. Funding There is no interhospital billing for tests. The budget for the participating hospital laboratories is administered globally and new programs or relocation of test facilities are funded by budget transfer between the appropriate hospitals. Data Sharing Model Manual orders and results connect tests across the two hospitals. Referred in tests from HRLMP clients to HHS site labs use bidirectional HL7 interfaces for orders/results. A set of Medinet proprietary HL7 interfaces connect HHSC with West Lincoln, one to send orders and receive results at West Lincoln, the other to receive orders and send results at HHSC. Link to Diagram Click here for process diagram. LIS’s used Meditech Magic 5.5 at HHS. McKesson Star 8.0 at SJHC. HL7 Version 2.3 Network Connectivity Direct connection to a node on a common fibre backbone which is behind a firewall. Nomenclature The Meditech and McKesson Star LIS’s dictate test nomenclature in use at HRLMP. No standard nomenclature in place. QA Data/Patient Identifiers Barcoding is done at source. Connectivity Interfaces to client sites monitored daily. Nomenclature/Mapping Will be done during OLIS implementation Corrective Actions Ad hoc process as major issues arise. Comments on Privacy & Security Controlled access to LIS. Network is firewalled. 43 11.7. Hamilton Regional Laboratory Medicine Program How SSHA Fits in the Picture Hamilton Regional Laboratory Medicine Program (HRLMP) As of August 2006 No role at this time How OLIS Fits in the Picture TBD, As of July 2007, the Meditech LIS will be the sole LIS in use at the HRLMP. When OLIS is implemented, it will be implemented to the Meditech LIS. Critical Success Factors The HRLMP, a regional academic health sciences laboratory, has a single administrative structure serving two acute care hospital corporations, a laboratory reference centre, and several outpatient specimen collection centres. This single structure is critical in aligning the efforts of the Program in service, research and academic pursuits. Another critical factor is the availability and application of technology in support of data sharing between the HRLMP and its customers. Vendor support of technology and interfaces facilitate data sharing. Dedicated LIS and IT staff to support interfaces and data sharing functionality. Iterative development of the Medinet interface. Benefits of This Initiative Provides service on a regional basis. Rationalizes the provision of expensive or complex tests. Eliminates inter-hospital billing for tests. Contact Info Christine Probst Manager, Laboratory Information Systems Hamilton Regional Laboratory Medicine Program probst@hhsc.ca 905-521-2100 x77517 44 HRLMP Process Diagram Electronic connection Manual/physical connection Clinician Clinician Orders/Results Orders/Results Hamilton HSC (Meditech LIS) Manual Orders/Results Same model is used for all client sites St. Joseph’s HC (McKesson Star LIS) Specimens HL7 Interface Orders/Results Medinet HL7 Interface West Lincoln Memorial Hospital (Meditech LIS) HICL (Proprietary LIS) Clinician 45 Canadian Medical Laboratories 11.8. InterHospital Laboratory InterHospital Laboratory Partnership (IHLP) Partnership As of August 2006 Lab Reform Region South West Description Regional integration of laboratory services of 6 member organizations across 11 hospital sites. Category Multiple LIS Vendor/Multiple System Integration Extent of Sharing 4 member sites have fully integrated LIS with main referral lab at Stratford site of Huron Perth Healthcare Alliance (HPHA). Other member sites and the community share referred test orders/results and specimens. Pathology reports are shared with CCO. Status/Timeframe Implemented project. Organizations Involved InterHospital Laboratory Partnership membership list. CCO. Governance Model Organizations have their own board and CEO Planning is done by steering committee and input from all parties involved including IHLP Management, which are all of the CEO’s of the different Hospitals. Funding All tests referred into Stratford are billed based on an IHLP fee-for-service formula that is approved by all Hospital CEO's yearly. Data Sharing Model 4 member sites are integrated with Stratford referral lab’s Meditech database. Listowel site has a Medinet interface for referred orders and results. Other sites use manual orders and receive faxed results for referred tests. Link to Diagram Click here process diagram. LIS’s used Meditech 4.9, Medinet. NPR report (for CCO reports) HL7 Version 2.3 Network Connectivity Modem to Modem asynchronous serial connection ( HICL & JBMH ) All connections including the MEDINET INTERFACE use a (comcentric pipe) which is a private healthcare pipe. Nomenclature No nomenclature standards. Once OLIS has been implemented, LOINC will be used for OLIS transactions. QA Nomenclature/Mapping LIS dictionary audits monthly. Connectivity Interfaces are checked on a yearly basis. Data Reports are checked on a yearly basis. Calculation verification checks. Policies on registration, manual order entry, faxing, etc. Corrective Actions Centralized Help Desk at the Stratford site, with escalations to application specialists and LIS coordinator. 46 11.8. InterHospital Laboratory InterHospital Laboratory Partnership (IHLP) Partnership As of August 2006 Comments on Privacy & Security Controlled access to the Meditech LIS and Medinet. Member organizations have their own policies on privacy & security. Network is firewalled. How SSHA Fits in the Picture TBD How OLIS Fits in the Picture TBD Critical Success Factors Physician and staff buy in. Benefits of This Initiative Improved turnaround times. Minimized transcription errors. Improved work flow. Saved adding staff for volume of work. Allows staff to move from one site to another with minimal training. Contact Info Paul Brown Lab Manager Huron Perth Healthcare Alliance Stratford Site paul.brown@hpha.ca 519-272-8210 x2892# Participating IHLP Members • Alexandra Marine & General Hospital (AM&G) • Huron Perth Healthcare Alliance –(Clinton, Seaforth, St. Marys & Stratford Sites) (HPHA) • North Wellington Health Care Corporation – (Mt. Forest & Palmerston Sites) • Listowel Wingham Hospitals Alliance – (Listowel & Wingham Sites) • South Huron Hospital – Exeter • Hanover & District Hospital 47 IHLP Process Diagram Clinician Electronic connection Manual/physical connection Sites w. Meditech LIS* Listowel Medinet HL7 Interface orders/results Shared Database HPHA Stratford Site (STO Meditech LIS and Database) orders/results NPR Report Specimens Lab Staff Manual/Faxed orders/reports Clinician (NonMeditech Site)** ** Exeter, Hanover, Mt. Forest, Palmerston, Wingham 48 CCO (PIMS) Specimens Specimens * Alexandra Marine & General, HPHA Clinton, Seaforth and St. Mary’s sites 11.9. London Laboratory Services Group London Laboratory Services Group (LLSG) As of August 2006 Lab Reform Region South West Description Centralized repository of patient information with integrated LIS modules among Thames Valley District hospitals including two reference laboratories at London Health Sciences Centre (LHSC) and St. Joseph’s Health Care (SJHC). Category Single LIS Vendor/Single System Integration Extent of Sharing Full sharing of LIS across sites with referred lab services to reference lab sites. Pathology reports are shared with CCO. Referred in orders and results are interfaced directly with Hospitals In Common Laboratory (HICL) Toronto. Status/Timeframe Project implemented for sharing of LHSC/SJHC LIS with Alexandra Hospital in Ingersoll, Four Counties Health Services in Newbury and Strathroy Middlesex General Hospital. Remaining Thames Valley District hospitals’ implementation anticipated for 2006. Organizations Involved 8 member hospitals of Thames Valley District (see list). CCO. HICL. Governance Model Joint Management Committee with representation from each of the Thames Valley Hospitals. A Memorandum of understanding has been signed by Thames Valley Hospitals for each of the major IT Projects. Funding Phase 1 – Rural & Northern Initiatives provided a $1.3 million grant to extend the Cerner PathNet LIS to the initial hospitals. Phase 2 – Funds for remaining integration will come from within the hospitals global budgets or reserve funding. Ongoing Support – from within the hospitals global budget. Data Sharing Model Currently, orders are handled through an order transcription process (orders written in the chart and transcribed by a unit clerk or nurse) but work is underway to introduce Clinical Provider Order Entry. Clinical areas access a centralized clinical information system, and specimens are routed accordingly by the system. Results are reported directly into the system. Link to Diagram Click here for process diagram. LIS’s used Cerner PathNet Millenium System 2003.02 HL7 Version 2.2,2.3 Network Connectivity Connected via VPN tunnels over Bell’s Core Network Nomenclature No nomenclature standards. Cerner PathNet system is capable of supporting LOINC, ICD-10 and SNOMED. Decisions on nomenclature and coding standards are pending direction from OLIS and CCO. QA Before and after each data conversion data is examined to ensure integrity of the conversion. Data/Patient Identifiers 49 11.9. London Laboratory Services Group London Laboratory Services Group (LLSG) As of August 2006 Connectivity Network devices are tested continually to ensure appropriate transmission of data. Interfaces have built-in error checking (AC/NAC) to ensure the data transmission maintains integrity. Nomenclature/Mapping For the most part, data mapping tables are not required as part of the Cerner computer system (since Cerner uses a Central Repository of data which multiple departments use for storing and accessing data). For data transferred across interfaces to foreign systems, conversion tables are used to map data to appropriate data fields in the foreign system. These tables are used by the Cerner Open Engine interface engine to ensure the various computer systems receive the appropriate data for their purposes. Corrective Actions LHSC has a team of programmers dedicated to supporting IT needs including interfaces. They work collaboratively with other agencies affected by the interfaces. Comments on Privacy & Security LIS access is controlled. Network is firewalled. Strict controls on network access from remote locations using data tokens. The Privacy Office conducts ongoing audits of access to patient data to ensure only legitimate access to patient data is respected. How SSHA Fits in the Picture TBD How OLIS Fits in the Picture TBD Critical Success Factors Common vision. Persistence. Corporate direction. Benefits of This Initiative Easier to manage data and compare productivity indicators. Ensures standards and procedures are consistently adhered to. Contact Info Dr. Robert Lannigan Clinical Microbiologist London Health Sciences Centre robert.lannigan@lhsc.on.ca 519-685-8213 Participating Thames Valley District Hospitals • Alexandra Hospital in Ingersoll • St. Thomas Elgin General Hospital • Four Counties Health Services in Newbury • Strathroy Middlesex General Hospital • London Health Sciences Centre • Tillsonburg District Hospital • St. Joseph’s Health Care London • Woodstock General Hospital 50 LLSG Process Diagram Electronic connection Manual/physical connection Client Site Transcribed Orders Specimens Results Cerner Integrated Clinical Information System Orders/ Results Specimens Cerner Open Interface Engine Reference Lab Pathology Reports Orders/ Results CCO (PIMS) HICL (Proprietary LIS) 51 Same model is used for all client sites 11.10. Mount Sinai Hospital Mount Sinai Hospital (MSH) As of August 2006 Lab Reform Region Toronto Description MSH acts as a reference lab for certain Toronto clients, setting up electronic interfaces for them, as well as sharing microbiology services with Toronto Medical Laboratories (TML). Category Multiple LIS Vendor/Multiple System Integration Extent of Sharing Full sharing of orders/results and specimens for referred in tests. Full sharing of microbiology services with TML/University Health Network (UHN). MSH shares pathology reports with CCO (not depicted). Status/Timeframe Implemented project. Organizations Involved MSH, Toronto Rehabilitation Institute, TML/UHN, CCO, Rouge Valley Health System (RVHS), Baycrest Centre for Geriatric Care, Humber River Regional Hospital (HRRH), Hospitals In Common Laboratory. Governance Model The Laboratory information system (LIS) at Mount Sinai hospital is jointly administered by the Microbiology department and the PLM department. Each department has 2 LIS officers committed full-time to the administration of the LIS. HP, through a contract with Mount Sinai hospital, supports the infrastructure for all informatics including the LIS. Funding Funding for referred-in tests is either based on a contractual agreement or fee for service. For the shared microbiology service, fee for service invoices are charged for referred-in tests, while the operating budget is shared 66:34 between UHN and MSH. Data Sharing Model LIS connects to different sites via several interface engines Link to Diagram Click here for diagram of data sharing model LIS’s used Soft Computer Consultants, Inc. (SCC), Meditech. HL7 Version HL7 2.3 Network Connectivity VPN over internet and ISDN lines Nomenclature No set nomenclature standards. Will meet OLIS nomenclature standards as required by project implementation. QA Data/Patient Identifiers In accordance with CAP and OLA standards, data transmissions are checked for end to end accuracy once a year and with upgrades to LIS software. Connectivity The network is monitored 24X7 by HP. The interface engines are monitored 24X7 by the respective vendors (Cloverleaf and MDI). Interfaces are checked daily by LIS officers 52 11.10. Mount Sinai Hospital Mount Sinai Hospital (MSH) As of August 2006 Nomenclature/Mapping Mapping is used for only 1 client (HRRH) and we rely on the client to report any mapping issues. Corrective Actions LIS officers are available 24X7 to correct, on an as needed basis, any issues that may arise. Comments on Privacy & Security MSH LIS modules are accessible only by the use of a user ID/password combination which is encrypted. User access is controlled internally. Client organizations follow their own privacy & security policies. How SSHA Fits in the Picture n/a How OLIS Fits in the Picture Plans to integrate with OLIS TBD. Critical Success Factors Establishing standard connectivity processes. Clear understanding of interface specifications by all parties involved. The use of interface engines has greatly facilitated interfacing efforts. Benefits of This Initiative Less paper, less transcription therefore less data entry work. Improved accuracy. Improved turnaround time. Contact Info David McPherson Senior LIS Officer, Pathology & Laboratory Medicine Mount Sinai Hospital 416-586-4800 ext.1597 dmcpherson@mtsinai.on.ca 53 MSH Process Diagram Electronic connection Clinician Clinician Clinician Manual/physical connection Baycrest (Meditech LIS) MSH (Cerner) HRRH Specimens Specimens Specimens Orders/Results Cloverleaf Interface Engine MSH Main Lab (SCC) MSH/TML Micro. Shared Service Lab (SCC) Orders/Results Micro. Orders/Results Orders/Results E-Gate Interface Engine Orders/Results RVHS Custom Interface RVHS (Meditech LIS) UHN (MISYS) Clinician Clinician 54 Microbiology Specimens Specimens Orders/Results 11.11. North Eastern Ontario Network North Eastern Ontario Network (NEON) As of August 2006 Lab Reform Region North East Description Integrated multi-site lab information system Category Single LIS Vendor/Single System Integration Extent of Sharing Full sharing of laboratory information. Orders/results are also shared on a referral basis with HICL (not depicted). Information is shared with eCHN (not depicted). Labs within NEON also act as back-up sites for other NEON labs. Status/Timeframe Implemented project. Organizations Involved 9 member hospitals in the region (see list). Governance Model The governance structure is a joint partnership, with a Steering Committee comprised of members from each organization. The present voting mechanism for decision making is one equal weighted vote per member. Funding The financial model is a cost-sharing mechanism, based on the cost of hardware, support, software, etc. The costs are allocated by member site, according to a bed formula (presently under review). An annual budget is created and approved through the steering committee. The initial start up costs for hardware, software and installation (incurred about 5 years ago) were shared amongst the members using the same bed allocation formula. Each year a budget is prepared and presented to NEON Steering for approval. The operational costs, including 16 shared FTE support staff centered in Sudbury, are also shared using the cost sharing formula. Data Sharing Model Regional information is accomplished through the use of a shared version of Meditech Client-Server. The main system resides in a single location and integration of information across all active modules is seen. Through NEON, smaller organizations were afforded an opportunity to implement the MEDITECH environment in a cost effective manner. Link to Diagram Click here for the process diagram. LIS’s used Meditech Client Server 5.3 HL7 Version 2.3 Network Connectivity All connectivity runs over Bell/SSH MPLS Network. Nomenclature Integrated architecture and a shared data base structure ensure a common internal nomenclature. Standard nomenclatures are considered, such as LOINC, but not strictly adhered to within the dictionaries. QA Data/Patient Identifiers All patients in the shared database are assigned a Unique Identifier in the system. This number remains with the patient and is used system wide. Connectivity Data lines are monitored continuously by vendor. 55 11.11. North Eastern Ontario Network North Eastern Ontario Network (NEON) As of August 2006 Nomenclature/Mapping Dictionary changes can occur at anytime required and the process is to test the change in the “test” environment prior to live implementation. Corrective Actions Sudbury support staff (shared FTE’s) will help address these issues. Each module has a core team of users, with membership from the various sites, to help identify and correct problems. Comments on Privacy & Security Security/Confidentiality addressed through policies and recognition of NEON as being an extended “circle of care”. Access to the LIS is controlled by username/password access and through staged level of access by job description. How SSHA Fits in the Picture Network connectivity provider. How OLIS Fits in the Picture TBD Critical Success Factors Strong leadership and vision from the participating organizations. Clearly defined benefits of integration. Varied organizations starting points for the membership to account for different stages of HIS/LIS development. Integrated database for clinicians was the prime focus. Benefits of This Initiative Allowed for the Meditech architecture to be afforded to smaller organizations. The shared costing model helps to spread out the cost of implementation and ongoing operational costs. The interconnectivity of the LIS module allows for reference testing to be done seamlessly, with on-line batching of reference tests and immediate distribution of verified results to clinicians, through the EMR. In addition, labs can act as each other’s back-up in case of production downtime. Contact Info Derek Graham Diagnostic Imaging Temiskaming Hospital dgraham@temiskaming-hospital.com 705-647-1088 x2170 Participating NEON Member Hospitals • Hôpital Regional De Sudbury Regional Hospital • Temiskaming Hospital • Englehart and District Hospital Inc. • Timmins And District Hospital - L’hopital De Timmins Et Du District • Kirkland And District Hospital • Northeast Mental Health Centre • Services De Sante De Chapleau Health Services • Smooth Rock Falls Hospital Corporation • St. Joseph’s General Hospital Elliot Lake 56 NEON Process Diagram Electronic connection Manual/physical connection Specimens Clinician Orders/results Specimens Orders/results Meditech Database Orders/results HICL Other NEON Site Lab (as required) NEON Site Lab 57 Same model is used for all client sites 11.12. Northwest Health Network Northwest Health Network (NWHN) As of August 2006 Lab Reform Region Northwest Description Regional integration of IT services under a single system Category Single LIS Vendor/Single System Integration Extent of Sharing Full sharing of orders/results and specimens which are sent to referral labs (MDS and Thunder Bay Regional Health Sciences Centre [TBRHSC]). Status/Timeframe Shared health information system currently implemented at 7 of 11 NWHN hospitals. Proposed project to implement shared LIS modules. Anticipating 2005-06 integration of remaining NWHN hospitals. Anticipating 2006-07 implementation of lab modules. Organizations Involved TBRHSC, St. Joseph’s Care Group (SJCG), 9 other referring hospitals (see list), MDS. Governance Model Individual hospital project teams report to a regional IS Project Steering Committee. Each organization governs its own lab services. Funding A portion of each hospital’s global budget funds this project, via a funding formula. Referred tests are compensated fee-for-service. Data Sharing Model MDS is main regional referral lab while some hospitals send certain tests to TBRHSC. Tests sent by referring hospitals are accompanied by written orders for manual data entry into testing lab’s LIS. TBRHSC has an interface with MDS. Results from TBRHSC are entered directly into regional Meditech HIS. Results from MDS are sent via interface to TBRHSC and manually faxed to other referring hospitals. TBRHSC and SJCG share IT services. Link to Diagram Click here for process diagram. LIS’s used Meditech Client Server. MDS uses their own proprietary system HL7 Version 2.2 Network Connectivity Data transmissions use SSHA pipelines. Nomenclature n/a QA Data/Patient Identifiers n/a Connectivity For MDS interface, data transmissions were tested prior to “go live”. Testing process included verification of sending and receiving of orders and results at reference and client labs. Abnormal and critical flagging, profile, group and reflex test order scenarios were all checked. Transmission logs are reviewed daily for failed transmissions. Nomenclature/Mapping Updates to mapping tables shared on a monthly basis. 58 11.12. Northwest Health Network Corrective Actions Northwest Health Network (NWHN) As of August 2006 TBRHSC help desk handles first tier support. Additional issues for the MDS interface may require direct communication (phone and email) between LIS coordinator, locale MDS office and MDS IT support. Comments on Privacy & Security How SSHA Fits in the Picture Controlled access to LIS. How OLIS Fits in the Picture TBD Critical Success Factors Agreement on a single LIS platform. Network connectivity provider. Organizational buy in. Development of a global funding formula Benefits of This Initiative Regional access to patient lab data. More efficient use of lab services. Contact Info Bev Junnila Technical Director - Laboratory Services Thunder Bay Regional Health Sciences Centre junnilab@tbh.net 807-684-6000 Participating NWHN Hospitals • Nipigon District Memorial Hospital • The McCausland Hospital • Geraldton District Hospital • Manitouwadge General Hospital • Wilson Memorial General Hospital • Atikokan General Hospital • Red Lake Margaret Cochenour Memorial Hospital • Dryden District General Hospital • Sioux Lookout Meno-Ya-Win Health Centre 59 NWHN Process Diagram Electronic connection Manual/physical connection Clinician Faxed Results Client Site (Meditech Client) Faxed Orders MDS Lab Staff Specimens Specimens Orders/Results Regional Meditech Database MDS (Proprietary LIS) Orders/Results Custom Interface Orders/Results Thunder Bay RHSC (Shared Meditech LIS) St. Joseph’s Care Group (Shared Meditech LIS) Clinician Clinician Specimens 60 Typical model for NWHN sites with implemented regional Meditech 11.13. Pathology Information Management System Pathology Information Management System (PIMS) As of August 2006 Lab Reform Region All Description Electronic identification and transmission of cancer case pathology reports from labs to Cancer Care Ontario (CCO). Category Province-wide Data Repository Extent of Sharing Full sharing of complete cancer pathology reports from hospital to CCO. Status/Timeframe Implemented project. PIMS roll-out took place from 2003-2005 with the main project completion in April 2005; MDS Labs was added in July 2006. Organizations Involved 45 provincial labs (representing 90% of the provincial pathology reports) – majority hospital labs (see attached list of members). Other hospitals still submitting but on disk or via fax Governance Model Project is a CCO initiative. Funding Following the CCO Information Management Strategy, MOHLTC funding under DTRACC (Data Tracking, Referral and Analysis of Capacity for Cancer Care) Data Sharing Model Pathology reports are filtered by locally installed client software for cancer cases, and then shared via unidirectional interface with CCO. Some implementations provide a central filter where the reports are sub-divided at CCO. Link to Diagram For Generic PIMS Architecture Diagram click here. LIS’s used Each site runs its own LIS, which may have a separate vendor for its pathology module. Those interfaced with PIMS include Eclipsys LabVision, Meditech Client Server, Meditech, Meditech Magic, SCC SoftLab, Cerner Classic, Cerner-CoPath, McKesson HBOC STAR, MISYS-CoPath and Intellipath. HL7 Version Ranges from 2.1 to 4.9 (majority approx. 2.6) Network Connectivity SSHA or via encrypted public internet (VPN). Nomenclature n/a – this project did not address this issue QA QA performed by CCO Coders as they review reports submitted. Data/Patient Identifiers 61 11.13. Pathology Information Management System Pathology Information Management System (PIMS) As of August 2006 Connectivity No automated central monitoring utility for PIMS sites in use at this time; CCO IT Operations Analyst runs a Crystal Reports inquiry daily to see which hospitals/labs have transmitted reports for the previous day, and to check the volume of reports. When a hospital stops transmitting reports for several days or has a significant reduction in the regular volume of reports over several days, the IT Ops Analyst contacts the hospital/lab to investigate the issue and resolve it if possible. If the issue cannot be resolved, the primary support vendor is contacted (see Corrective Actions). Nomenclature/Mapping n/a Corrective Actions If significant problems are encountered with the transmissions from a hospital/lab, the IT Ops Analyst will investigate and subsequently involve the primary PIMS vendor (Artificial Intelligence in Medicine – AIM) if required for correcting any major problems found. Comments on Privacy & Security Cancer Act governs CCO’s access to this data for maintaining the Ontario Cancer Registry. Compliant with PHIPA. How SSHA Fits in the Picture SSHA is the network infrastructure provider How OLIS Fits in the Picture Ultimately, OLIS will provide the service required by CCO as it will create and house a repository of all cancer pathology reports which CCO can access. However, this is a long-term plan and not likely to materialize in the next few years. Critical Success Factors Ability of technology to filter cancer from non-cancer reports. Understanding and Commitment of local pathology information management teams. MOHLTC support. Project Management Methodology and Rigorous application. Benefits of This Initiative Substantial improvements in overall data quality. Unprecedented capability for automated cancer surveillance. Enables of rapid enrolment of patients in to research studies. Significant cost reductions achieved. Traceability of disclosures for hospitals. Contact Info Andrea MacLean Pathology Project Manager Cancer Care Ontario andrea.maclean@cancercare.on.ca 416-217-1391 Pat Mason Pathology Project Consultant Cancer Care Ontario patrick.mason@cancercare.on.ca 416-971-9800 X3309 62 Participating PIMS Labs (as of August 2006) • Brantford General Hospital • Grand River Hospital Corporation • Kingston Hospitals Laboratories Services • London Health Sciences Centre • Joseph Brant Memorial Hospital • Thunder Bay Regional • Toronto East General • Hamilton Health Sciences Corporation • North York General • Humber River Regional • Mount Sinai • University Health Network • St. Joseph’s Toronto • Trillium Health Sciences • St. Michael’s Hospital • Halton Healthcare Services Corporation • St. Joseph’s London • Lakeridge Health Corporation - Oshawa • Queensway Carleton • Sunnybrook Health Sciences Centre • Stratford General Hospital • Rouge Valley Health System • Peterborough Regional Health Centre • Grey Bruce Health Services • Royal Victoria • Quinte HCC • Cambridge Memorial • William Osler • St. Mary’s • Ottawa Civic • Guelph General Hospital • Ottawa Hospital • St. Joseph’s Hamilton • Sudbury Regional Hospital Corporation • Southlake Regional • Scarborough Hospital • York Central Hospital • Niagara Health System • Credit Valley • Bluewater Health - Sarnia • North Bay General • Gamma Dynacare • Timmins and District Memorial • MDS Labs • Markham Stouffville 63 Generic PIMS Architecture Diagram (local filter) Hospital Pathology Lab (Pathology LIS) Pathology Reports Autocode Filter Application PIMS IP Network TransMed EDI TransMed EDI 64 11.14. Sunnybrook Health Sciences Centre Sunnybrook Health Sciences Centre (SHSC) As of September 2005 Lab Reform Region Toronto Description SHSC’s hospital laboratory acts as a referral lab for clients in the North East Regional Group. Category Multiple LIS Vendor/Multiple System Integration Extent of Sharing Full sharing of orders/results and specimens for referred in tests. Sharing of pathology reports with CCO. Status/Time frame Implemented project, with ongoing plans to increase scope Currently transitioning connectivity to use SSHA network. Planning to link with North York General Hospital, Southlake Regional Health Centre and Hospitals In Common Laboratory – target completion Spring 2006. Planning integration of blood bank transfusion system with UHN – anticipated over 2006-07. Organizations Involved SWCHSC, York Central Hospital, Markham Stouffville Hospital, North York General Hospital and Southlake Regional Health Centre. SWCHSC refers some tests to Gamma Dynacare and vice-versa. Governance Model Cooperative initiative. Participants meet on a regular basis to discuss services, issues, strategy and planning. Funding Each site pays their own vendor and connectivity costs (connectivity will transition to SSHA). Model based on fee for service. Each member is responsible for their portion of the cost. Data Sharing Model SHSC’s LIS connects directly to external partners. Link to Diagram Click here for process diagram. LIS’s used MISYS v5.3.3.2.5 and CoPath v2.3b. Meditech, MISYS and HBO McKesson at client sites. Proprietary LIS’s at HICL and Gamma Dynacare. HL7 Version 2.1, 2.3, depending on vendor system Network Connectivity SSHA, VPN, ISDN Link, Dial Up Nomenclature Each Institution have their own unique mapping requirements, typically vendor negotiated QA Data/Patient Identifiers Once monthly perform random data checks. Connectivity Interface is monitored regularly. Dispatching endpoints are responsible for checking message dispatch. Nomenclature/Mapping Systems are stable, verification is done only when mapping enhancements are required. Corrective Actions In the event of a service interruption one of the endpoints will notify the other. Also QA is performed monthly to validate data. 65 11.14. Sunnybrook Health Sciences Centre Sunnybrook Health Sciences Centre (SHSC) As of September 2005 Comments on Privacy & Security Network is firewalled and access to the LIS is controlled; only LIS staff have external access How SSHA Fits in the Picture SSHA will be the network connectivity provider in future integrations How OLIS Fits in the Picture TBD Critical Success Factors Meditech (Vendor to Vendor) LIS to LIS agreement to standardized transaction formats to support each vendor’s requirements. HBO/McKesson was unable to accommodate micro’s discreet components. They simply populate with text. Benefits of This Initiative Reduce resource effort. Improved turnaround times. Standardized Test Dictionary. Less human intervention. Reduction in transcription across systems. Contact Info Murray Kaufman Consultant Sunnybrook Health Sciences Centre murray.kaufman@sunnybrook.ca 416-480-6100 x3192 66 SHSC Process Diagram Electronic connection Clinician Manual/physical connection Sunnybrook Electronic Patient Record System (OACIS/Dinmar) Orders/results Enterprise Interface Engine (EBIZ-SYBASE) Sunnybrook LIS (CoPath/MISYS) Microbiology orders (text dump) Orders/results HL7 Interface Client Hospital (HBO McKesson) Client Hospital (Meditech or MISYS) CCO (PIMS) 67 HICL (Proprietary LIS) Gamma Dynacare (Proprietary LIS) Specimens Specimens Pathology Reports 11.15. Toronto Medical Laboratories Toronto Medical Laboratories (TML) As of July 2005 Lab Reform Region Toronto Description Joint venture between a hospital lab and a private lab that provides lab services to client hospitals Category Multiple LIS Vendor/Multiple System Integration Extent of Sharing TML works in partnership with other hospitals to provide laboratory services to University Health Network (UHN) hospitals. TML also provides full lab services to the Ajax-Pickering and Centenary Sites for the Rouge Valley Health System (RVHS), which includes interfaced orders and results. Microbiology services are provided to Baycrest Centre and are shared with Mt. Sinai Hospital (MSH). Status/Timeframe Implemented project with plans to expand client list. Proposed projects for 2005-06 include interfaces with Sunnybrook Transfusion Medicine, Trillium Gift of Life Network and Hospitals in Common Laboratory. Organizations Involved TML, UHN, MSH, RVHS, Baycrest, MDS Governance Model The relationship is between TML and its clients as a contractual arrangement for a purchase of services. Services are provided to the clients. Governance decisions are made by TML. Funding TML bills a portion of the start up costs at inception, and then the remainder of the cost of contract is handled on a multi-year basis, with allowances made for increased or decreased payments based on volume thresholds. Data Sharing Model TML’s 5 LISs currently connected operate separately and conduct all crosscommunications through the E-Gate. The interface engine (E-Gate) is software developed by the Courtyard Group that sits on its own server that formats, logs and makes adjustments in all message that pass between the different LIS. MSH orders on Cerner are transmitted through MSH’s Cloverleaf interface gate connection directly into SoftMic. Link to Diagram Click here for diagram of current interfaces to TML. LIS’s used MISYS, Meditech, ULTRA and I-Net [GE Healthcare], CoPath [Cerner], Softmic [SCC], Hemocare [Mediware] HL7 Version HL7 2.1-2.3 (depending on the system and interfaces) Network Connectivity Baycrest’s connection uses the SSHA pipeline. For Bridgepoint (a client hospital that does not use an HIS) the SSHA connection is used to generate reports directly to their printers on their internal network. Other connections are run on VPN connections over the internet or on ISDN lines. 68 11.15. Toronto Medical Laboratories Toronto Medical Laboratories (TML) As of July 2005 Nomenclature SNOMED; generally nomenclature is a cross-reference table mapping between individual lab systems. QA Data/Patient Identifiers Institution medical record number or OHIP number gets transmitted through the whole process. An institution key is assigned to data in the E-Gate and that number is readable by all the different systems. A single barcode is maintained throughout process, except for specimens processed by Meditech LIS. A separate barcode is attached at the TML site for Pathology, Microbiology and Transfusion Medicine specimens. Connectivity Periodic checks of data integrity. Nomenclature/Mapping Verified as changes require. Corrective Actions When isolated incidents occur, error logs are checked and the error is corrected after it is reported. Comments on Privacy & Security Access to LIS’s is controlled within TML. Clients follow their own privacy & security policies. How SSHA Fits in the Picture Network connectivity provider for certain clients. How OLIS Fits in the Picture Plans to integrate with OLIS TBD. Critical Success Factors Standardization of certain aspects of systems involved. The ability to avoid over-labeling created restrictions on the system (every system needs to be identified uniquely). Standardization also meant a large amount of field to field mapping and cross-referencing test names. Physical logistics of moving specimens from client sites to the laboratory site. Implementing data connections (a dedicated T1 line to RVHS, a VPN set up with MDS, etc.) A key to success was TML’s ability to generate cost savings for clients by capitalizing on its capacity (reducing TML’s own cost per test in the process). This provided the business case for clients to use this service. Benefits of This Initiative 80% of TML’s work comes through interfaces. The information exchange is vital to the continued success of TML. Order entry and results reporting is conducted in a timely manner. Extensive data mining is possible using the databases of the individual lab systems and, for UHN, indirectly through their MYSIS HIS. The interface model is a selling point for future partnerships by allowing for reduced front end workload without the need for over-labeling. Contact Info Peter Woo Manager, Information Systems, Toronto Medical Laboratories Peter.Woo@uhn.on.ca 416-340-4800 x2518 69 TML Process Diagram Electronic connection Clinician Clinician Clinician Clinician Manual/physical connection UHN (MISYS HIS) Rouge Valley HS Ajax-Pickering Site (Meditech LIS) Rouge Valley HS Centenary Site (Meditech LIS) Baycrest (Meditech LIS) Orders/Results Orders/Results E-Gate Interface Engine (Courtyard Group) Orders/Results Orders/Results TML General Lab (ULTRA) TML Pathology (CoPath) MSH/TML Shared Services Microbiology Lab (SoftMic) TML Transfusion Med (Hemacare) 70 Specimens Specimens RVHS Custom Interface 11.16. Trillium Health Centre Trillium Health Centre (Trillium) As of August 2006 Lab Reform Region Central West Description Fully integrated multi-site hospital laboratory. Category Single Vendor/Single LIS Integration Extent of Sharing In house testing has fully integrated data exchange. Referred out tests follow a manual process. Status/Timeframe Implemented project. Planned OLIS integration. Organizations Involved Trillium Health Centre (Mississauga and Queensway sites). CCO (not depicted). OLIS (planned). Governance Model All sites are governed under one corporation. Funding Lab services are funded by one corporation. Information systems are funded under yearly contracts, with ongoing operational costs for fixes, interfaces and upgrades. Data Sharing Model Full sharing of lab services between campuses. Manual orders and results process for referred out tests. Planned unidirectional feed to OLIS data repository. Planned integration between OLIS Viewer and Meditech LIS. Link to Diagram Click here to view process diagram. LIS’s used Meditech 4.9, upgrading to 5.5 in Sept. 2006. HL7 Version Not available Network Connectivity SSHA pipeline Nomenclature LOINC for OLIS, ICD-10 for Health records. QA Data/Patient Identifiers Barcoding being examined prior to implementation of physician order entry. Connectivity Functionality is tested daily by staff using the equipment. Nomenclature/Mapping Mapping tables being created and reviewed for OLIS, Eclipsys Sunrise Clinical Manager and Meditech. Semi-automated updating of table values in Meditech. Change control forms ensure sign off from all parties when changes are made. Corrective Actions Test suites are in place to define action on defect tracking and review of interface engine error logs. Meditech support and defect tracking system available to address issues. Comments on Privacy & Security How SSHA Fits in the Picture Controlled access to LIS, network firewalled. Network connectivity provider. 71 11.16. Trillium Health Centre Trillium Health Centre (Trillium) As of August 2006 How OLIS Fits in the Picture Trillium is an OLIS foundation adopter. Once implemented, clinicians can access historical results from the OLIS data repository via historical lookups from the Meditech LIS. Critical Success Factors Participation in OLIS foundation adopter program. Funding. Organizational buy in. Benefits of This Initiative Integration with OLIS for historical lookups will bring the following expected benefits: Contact Info • Reduce unnecessary duplicate laboratory testing • Provide timely access to patients’ laboratory records • Provide access to decision support and “best practices” information • Provide better data for policy analysis and business planning • Provide pseudonymous planning and management data • Provide access to research and analysis tools Andre Bottis Project Support Consultant, Information Technology Trillium Health Centre abottis@thc.on.ca 905-848-7580 x5725 72 Trillium Process Diagram Electronic connection Manual/physical connection Clinician OLIS Orders & Results Orders/results Orders/results Specimens OLIS Database Meditech Database Historical Lookups Orders/results OLIS Viewer Mississauga Site Lab Queensway Site Lab 73 11.17. William Osler Health Centre William Osler Health Centre (WOHC) As of August 2006 Lab Reform Region Central West Description Internal information exchange using a single health information system (Meditech) across multiple campuses. Category Single LIS Vendor/Single System Integration Extent of Sharing Full sharing of lab services between campuses. Status/Timeframe Implemented project. Organizations Involved WOHC consists of two hospital sites at Etobicoke, Peel Memorial and shares services with Georgetown Hospital. Governance Model All sites are governed under one corporation. Funding Lab services are funded by one corporation. Data Sharing Model Full sharing of lab services between campuses. Bi-directional exchange with HICL. Sharing of pathology reports with CCO (not depicted) Link to Diagram Click here to view process diagram LIS’s used Meditech version 4.9. HICL uses a proprietary LIS. HL7 Version 2.3 Network Connectivity SSHA/VPN/Fibre Nomenclature ICD 10 QA Data/Patient Identifiers Bar-coding of all specimens except pathology. Connectivity Internal monitoring Nomenclature/Mapping Edits from one party are communicated with other – on going process. Dictionary edits in Meditech take effect immediately after fully tested. They are automatically incorporated or copied to new updates or releases Corrective Actions IT initiates a Help Desk ticket immediately upon notification of a malfunction. This ticket is electronically updated as staff work to rectify the problem. The electronic ticket is closed once the problem has been resolved. Comments on Privacy & Security LIS is password protected and access controlled. Regular audits are conducted on firewall logs. How SSHA Fits in the Picture Network connectivity provider How OLIS Fits in the Picture TBD 74 11.17. William Osler Health Centre William Osler Health Centre (WOHC) As of August 2006 Critical Success Factors Bar-coding. Funding. Benefits of This Initiative Reduction in transcription errors. Broader availability of information. Real time access to information. Reduction in clerical time Contact Info Debbie Cock William Osler Health Centre debbie_cock@oslerhc.org 416-747-3400 x.32024 WOHC Process Diagram Electronic connection Manual/physical connection Specimens Clinician Orders/results Specimens Orders/results Meditech Database Orders/results HICL Peel Memorial Site Lab Etobicoke Site Lab 75 Georgetown Site Lab