Professor Kathleen V. Kilway and Robert Clevenger
advertisement
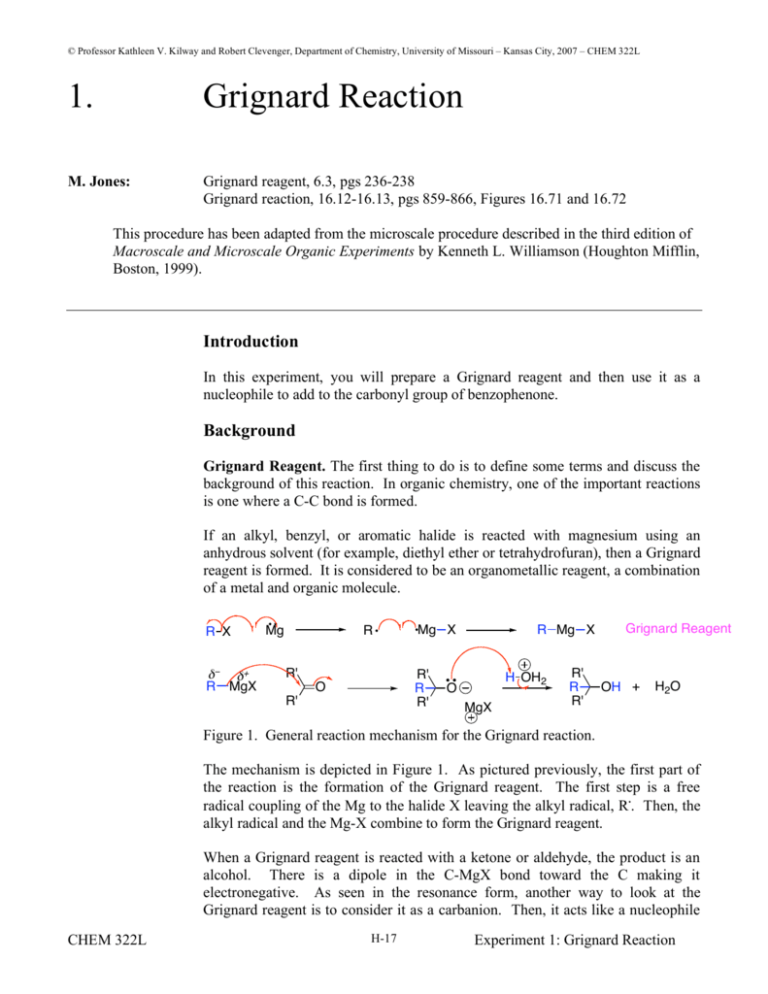
© Professor Kathleen V. Kilway and Robert Clevenger, Department of Chemistry, University of Missouri – Kansas City, 2007 – CHEM 322L 1. Grignard Reaction M. Jones: Grignard reagent, 6.3, pgs 236-238 Grignard reaction, 16.12-16.13, pgs 859-866, Figures 16.71 and 16.72 This procedure has been adapted from the microscale procedure described in the third edition of Macroscale and Microscale Organic Experiments by Kenneth L. Williamson (Houghton Mifflin, Boston, 1999). Introduction In this experiment, you will prepare a Grignard reagent and then use it as a nucleophile to add to the carbonyl group of benzophenone. Background Grignard Reagent. The first thing to do is to define some terms and discuss the background of this reaction. In organic chemistry, one of the important reactions is one where a C-C bond is formed. If an alkyl, benzyl, or aromatic halide is reacted with magnesium using an anhydrous solvent (for example, diethyl ether or tetrahydrofuran), then a Grignard reagent is formed. It is considered to be an organometallic reagent, a combination of a metal and organic molecule. R X + R MgX Mg R R' Mg X R' R R' O R' R Mg X H OH2 O MgX R' R R' Grignard Reagent OH + H2O Figure 1. General reaction mechanism for the Grignard reaction. The mechanism is depicted in Figure 1. As pictured previously, the first part of the reaction is the formation of the Grignard reagent. The first step is a free radical coupling of the Mg to the halide X leaving the alkyl radical, R.. Then, the alkyl radical and the Mg-X combine to form the Grignard reagent. When a Grignard reagent is reacted with a ketone or aldehyde, the product is an alcohol. There is a dipole in the C-MgX bond toward the C making it electronegative. As seen in the resonance form, another way to look at the Grignard reagent is to consider it as a carbanion. Then, it acts like a nucleophile CHEM 322L H-17 Experiment 1: Grignard Reaction and adds to the carbonyl carbon forming an alkoxide. In the workup of the reaction, an acid is added so that the alkoxide is protonated to form the alcohol product. See Figure 2 for the general reaction. R MgX R X R Mg O C 1) RMgX R 2) H2O OH R R R 1) addition 2) protonation yields a 3o alcohol OH H R R 1) addition 2) protonation yields a 2o alcohol ether R MgX R O C 1) RMgX H 2) H2O Figure 1. General reaction for the Grignard reaction. In this experiment, you will be performing a Grignard reaction (see Figure 3). You will first synthesize the Grignard reagent starting from bromobenzene and magnesium using ether as the solvent. The reaction and thus the reagent are moisture sensitive so you will be using anhydrous ether. In the second step, you will be adding your Grignard reagent to benzophenone to form triphenylmethanol. Mg + Br Magnesium At.W. 24.31 anhydrous diethyl ether (ether) Phenylmagnesium bromide an intermediate but not isolated Bromobenzene bp 156 oC M.W. 157.02 density 1.491 g/mL O MgBr Phenylmagnesium bromide MgBr OH 1) ether 2) dilute HCl + Benzophenone mp 48-49 oC M.W. 182.22 Triphenylmethanol mp 160-163 oC M.W. 260.34 Figure 3. The overall reaction of this experiment. CHEM 322L H-18 Experiment 1: Grignard Reaction Experiment There are three parts to the experiment. Make sure to read and following the warnings listed in the procedure. The preparation of the Grignard reagent and reaction are exothermic. Grignard Reagent Preparation Add 230 mg of magnesium turnings and anhydrous ether (5 mL) to a large reaction tube. Add 1 mL of bromobenzene to the tube and gently grind the magnesium with a large glass rod. As the mixture becomes cloudy, look for signs of bubbling (refluxing). Once refluxing begins, add another 3 mL of bromobenzene and tightly cap the reaction vial. Swirl the contents periodically. Once refluxing has ceased, cautiously vent the tube. [Warning: Not slowly unscrewing the cap can result in the violent release of the reaction tube contents.] The reaction is completed when either the magnesium is all consumed or refluxing has ceased. Synthesis of Triphenylmethanol Then, add a solution containing 1.7 g of benzophenone dissolved in 4 mL of anhydrous ether by pipette. [Caution: This reaction is exothermic and rapid addition of the benzophenone can result in the violent release of the reaction tube contents.] Refluxing should commence after 1 mL of reagent is added. Continue to add the benzophenone at a rate to maintain a refluxing solution. Once all the benzophenone has been added, cap the tube and swirl the contents for two minutes. Reaction Workup Cautiously vent the tube and begin adding a 50% HCl solution to the contents of the reaction tube [Caution: This reaction is exothermic and rapid addition of the HCl solution can result in the violent release of the reaction tube contents.] Continue to add the HCl solution until the aqueous layer is clear and free of any magnesium. Remove the aqueous layer, dry with sodium sulfate, filter, and evaporate solvent down to approximately 2 mL on a hotplate located in the hood. Cool the contents using an ice-bath. Wash the solid with three 4 mL portions of petroleum ether, removing each yellow wash with a pipette fitted with a cotton plug. Recrystallize the solid from ethanol. Take an IR spectrum of the final product. CHEM 322L H-19 Experiment 1: Grignard Reaction