week 3: grignard reaction
advertisement
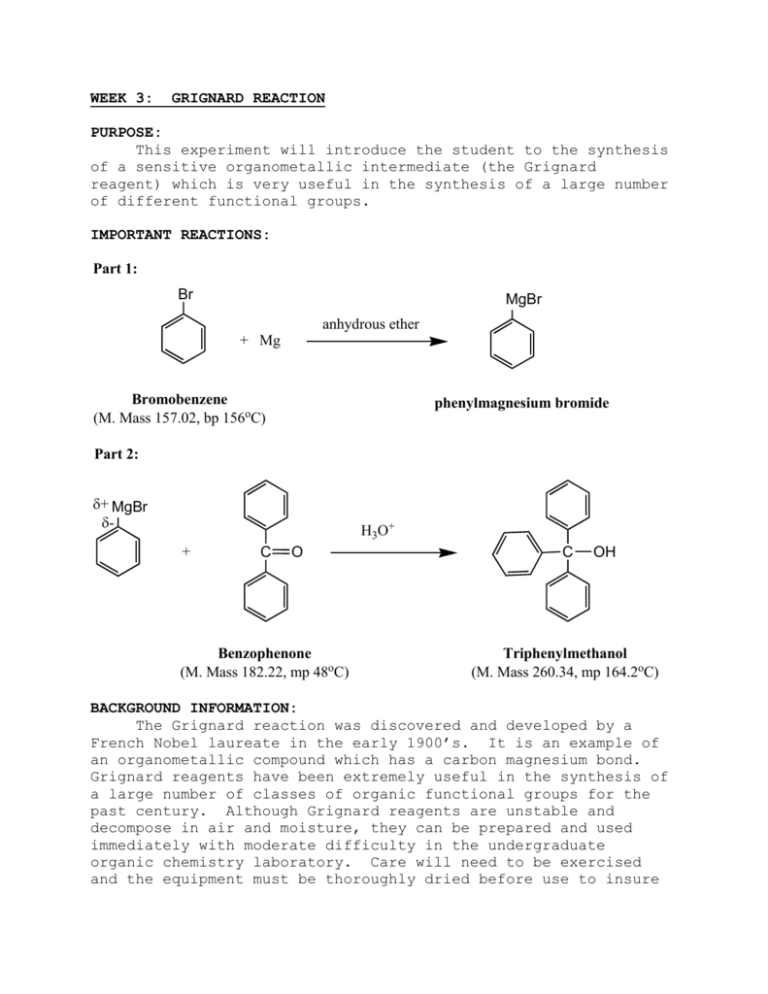
WEEK 3: GRIGNARD REACTION PURPOSE: This experiment will introduce the student to the synthesis of a sensitive organometallic intermediate (the Grignard reagent) which is very useful in the synthesis of a large number of different functional groups. IMPORTANT REACTIONS: Part 1: Br MgBr anhydrous ether + Mg Bromobenzene (M. Mass 157.02, bp 156oC) phenylmagnesium bromide Part 2: + MgBr - H3O+ + C O Benzophenone (M. Mass 182.22, mp 48oC) C OH Triphenylmethanol (M. Mass 260.34, mp 164.2oC) BACKGROUND INFORMATION: The Grignard reaction was discovered and developed by a French Nobel laureate in the early 1900’s. It is an example of an organometallic compound which has a carbon magnesium bond. Grignard reagents have been extremely useful in the synthesis of a large number of classes of organic functional groups for the past century. Although Grignard reagents are unstable and decompose in air and moisture, they can be prepared and used immediately with moderate difficulty in the undergraduate organic chemistry laboratory. Care will need to be exercised and the equipment must be thoroughly dried before use to insure success. No water may enter the reaction mixture until the final product has been synthesized. Group IA metals (Li, Na, and K) form very basic organometallic compounds which are very hazardous to handle. They can be spontaneously flammable in air and react explosively with water to release hydrogen gas. In these cases, the C-M bond is very polar and the carbon group is highly basic, especially in sodium and potassium compounds. With the Group IIA metal magnesium, the C-Mg bond is not as polar and the compound is not as difficult to handle. Yet they are reactive enough to be very useful in synthesis. The exact nature of the bonding in Grignard reagents has been extensively studied in the past century and is beyond the scope of this discussion. For the purposes of this experiment, one can consider the bonding to be R-MgBr, where R is an alkyl or aryl group. Organometallic compounds with transition metals (such as, iron, mercury, lead, tin, cadmium, and many others) have an even more covalent carbon metal bond. Many of these compounds are stable and often very toxic. Organic mercury compounds are often mentioned as environmental hazards in the New Jersey area. One reason for the extreme toxicity of some organometallic compounds is the volatility that the organic group imparts on the molecule which makes it easier for entry into the body. Returning to the chemistry of the organomagnesium (Grignard) compounds, they are generally prepared by the reaction of an alkyl or aryl halide with magnesium metal turnings. The magnesium turnings are crushed in a mortar and pestle just before use to expose a fresh surface on the metal. Of the halides used, iodides are best, fluorides are the least reactive and bromides are usually preferred for their good reactivity and lower cost. Grignard reagents are usually prepared in anhydrous ether as the volatile ether solvent helps keep air away from the reaction. It cannot be stressed enough that the ether must be very dry and kept that way if the reaction is to be successful. Small traces of water will destroy the reagent or keep the reaction from starting. Once formed, the Grignard reagent must be used immediately. One can react this reagent with a large number of different functional groups to give a wide variety of possible products. The Grignard reagent can react with: 1. any active hydrogen (such as water, alcohols, carboxylic acids, etc.) to produce and alkane or arene (usually not desirable). 2. formaldehyde to form a primary alcohol. 3. an aldehyde (other than formaldehyde) to form a secondary alcohol. 4. a ketone to form a tertiary alcohol (which will be done in this experiment). 5. an ester to form a tertiary alcohol. 6. carbon dioxide (as solid dry ice) to form a carboxylic acid. 7. many other classes of compounds to form other useful products. Due to the great reactivity of Grignard reagents, the alkyl or aryl halide starting material cannot have any reactive function group as part of its molecule. This generally limits the halide to be alkyl, alkenyl, alkynyl, or aryl. An ether group is also compatible and unreactive with the Grignard reagent. In today’s experiment, bromobenzene will react with magnesium turnings to form phenyl magnesium bromide (the Grignard reagent). This intermediate will then be immediately treated with benzophenone to prepare triphenylmethanol (a solid tertiary alcohol). EXPERIMENTAL PROCEDURE: In order to minimize the exposure of the Grignard reagent to water, the equipment and reagents used in this experiment must be absolutely dry. All glassware (two reaction tubes, two sample vials and a stirring rod) must be pre-dried in an oven at 110oC for at least 30 minutes prior to use. The anhydrous diethyl ether solvent and the bromobenzene will be stored over molecular sieves to absorb any moisture they are exposed to and the magnesium metal will be pre-dried in an oven. PART 1: Generation of Grignard Reagent Remove the first reaction tube from the oven and cap it with a rubber septum. Add magnesium powder (50mg, 2 mmol) to the tube, minimizing the time that the tube is un-capped. Carefully add anhydrous diethyl ether (0.5 mL) to the tube using a dry syringe, injecting the needle through the rubber septum. If a glass syringe is not available a dried disposable glass pipette can be used instead. Remove a sample vial from the oven and cap it with a rubber septum. To this vial add bromobenzene (330 mg, 2.1 mmol) and diethyl ether (0.7 mL) via the syringe as you did for the first tube. After addition of the ether solvent, do not remove the needle from the septum. As soon as the ether has been added to the vial, swirl the mixture and immediately remove the entire bromobenzene-ether mixture by carefully sucking it up into the syringe. Once this has been done, inject approx. one third of the bromobenzene-ether mixture into the first reaction tube containing the magnesium metal in ether. Mix the contents and add a pressure release needle to the septa. If the reaction does not appear to start at this point, first remove the syringe (if used) containing the remaining bromobenzene-ether mixture. Carefully remove the septa and the pressure release needle and quickly grind the magnesium metal using the oven dried glass stirrer rod. Replace the septa and pressure release needle as soon as possible to minimize air-water exposure. Once the reaction starts, the clear solution will become cloudy and the ether may begin to boil. At this point, reattach the syringe containing the remaining bromobenzene-ether mixture and add the rest of the solution dropwise at such a rate that the reaction does no go out of control. Once the entire contents of the syringe has been added, continue to agitate the reaction vessel until the reaction is complete, which will be visible when very little, if any, magnesium metal remains. The synthesized phenylmagnesium bromide is not isolated, but will be used in situ for part 2. PART 2: Synthesis of Triphenylmethanol Remove the second oven dried vial and cap it with a rubber septa. Add benzophenone (0.364 g, 2.0 mmol) and diethyl ether (1.0 mL) in the same manner and using the same precautions as you did in part 1. Shake the vial to dissolve the benzophenone and remove the resulting solution by sucking it up into the dry syringe. Carefully remove the syringe containing the benzophenoneether solution and inject it dropwise into the reaction tube containing the phenylmagnesium bromide. A red colour should be observed at this point. After all the benzophenone solution has been added, rinse the vial with a few drops of ether and inject these washings into the reaction tube. The reaction is complete when the red colour disappears. On completion, cool the reaction tube in ice, and very carefully add 3 M hydrochloric acid (2 mL) dropwise with stirring. If there is any un-reacted magnesium metal in your reaction tube be especially careful as acids react with metals to release hydrogen gas. This could lead to your reaction solution bubbling out of your tube. On addition of the acid, a two-phase system should result, add more ether solvent if the white triphenylmethanol product begins to crystallize out. Carefully remove the lower aqueous layer using a Pasteur pipette. To the remaining ether layer add an equal volume of saturated aqueous sodium chloride solution, shake the tube, allow the layers to settle and remove the lower aqueous layer as before. Dry the ether layer over calcium chloride pellets for 510 minutes, then carefully transfer the ether layer via pipette into a pre-weighed beaker. Wash the drying agent with a small amount of ether and combine the washings with the solution in the beaker. Remove the ether solvent by carefully blowing a stream of air over the solution in the beaker. When it appears to be dry, add ice-cold petroleum-ether (1 mL) to the white residue and grind (triturate) the product with a glass rod. Filter the product using a Hirsch funnel and air dry the solid. Re-weigh the beaker to record the mass of triphenylmethanol produced. Calculate the percent yield and determine the melting point. IMPORTANT INFORMATION ABOUT THE REPORT: The report for this experiment will follow the usual format. Be sure the percent yield calculation is carefully done. Also, record the melting point range of the final product and compare that melting point to the reported melting point of triphenylmethanol. Using these data, discuss the relative success on the experiment. END OF EXPERIMENT. © 2007 STEPHEN ANDERSON AND ROBERT SHINE