Chapter 8 (part 1)
advertisement

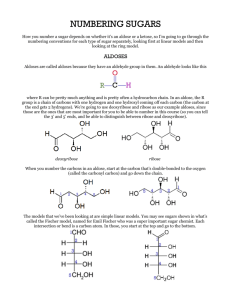
Chapter 8 (part 1) Carbohydrates Carbohydrates • Most abundant class of biological molecules on Earth • Originally produced through CO2 fixation during photosynthesis Roles of Carbohydrates • Energy storage (glycogen,starch) • Structural components (cellulose,chitin) • Cellular recognition • Carbohydrate derivatives include DNA, RNA, co-factors, glycoproteins, glycolipids 1 Carbohydrates • Monosaccharides (simple sugars) cannot be broken down into simpler sugars under mild conditions • Oligosaccharides = "a few" - usually 2 to 10 • Polysaccharides are polymers of the simple sugars Monosaccharides • Polyhydroxy ketones (ketoses) and aldehydes (aldoses) • Aldoses and ketoses contain aldehyde and ketone functions, respectively O H C CH2OH H C* OH HO C* H H C* OH • Ketose named for “equivalent aldose” + “ul” inserted • Triose, tetrose, etc. denotes number of carbons • Empirical formula = (CH2 O)n C O HO C* H H C* OH CH2 OH CH2OH D-ribul ose D-ri bose Monosaccharides are chiral • Aldoses with 3C or more and ketoses with 4C or more are chiral • The number of chiral carbons present in a ketose is always one less than the number found in the same length aldose • Number of possible steroisomers = 2 n (n = the number of chiral carbons) O H C CH 2OH H C* OH HO C* H C O HO C* H C* H OH H C* H C* OH OH H C* OH CH 2OH CH 2OH D-gl ucose D-fruct ose 2 Stereochemistry Enantiomers O H O C C HO C* H H C* OH HO C* HO C* Epimers D iastereomers O H O H C C C* OH HO C* H H C* OH HO C* H HO C* H HO C* H H C* OH H C* OH H O H H C* OH H C* OH CH 2OH CH 2OH D-gluc os e H O H C H L-glucose O H H C H C* OH HO C* H H HO C* H HO C* H C* H H C* OH H C* OH C* OH H C* OH H C* OH CH2OH CH 2OH D-mannos e D-galactose CH 2OH CH 2OH D-gluc os e D -mannose •Enantiomers = mirror images •Pairs of isomers that have opposite configurations at one or more chiral centers but are NOT mirror images are diastereomers •Epimers = Two sugars that differ in configuration at only one chiral center Cyclization of aldose and ketoses introduces additional chiral center • Aldose sugars (glucose) can cyclize to form a H cyclic hemiacetal H N EW CHIRAL A LDEHYD E O O C H ALC OHOL H R1 C* O R2 O H C ARB ON R1 R2 HEMIAC ETAL • Ketose sugars (fructose) can cyclize to form a H H cyclic hemiketal N EW CHIRAL KE TON E O O C ARB ON C R C* R1 R1 R O ALC OHOL R2 O H R2 HE MIKE TAL Glucopyranose formation 3 Fructofuranose formation Monosaccharides can cyclize to form Pyranose / Furanose forms α = 64% β = 36% α = 21.5% β = 58.5% α = 13.5% β = 6.5% Haworth Projections O H -OH up = beta -OH down = alpha C1 H C2 OH HO C3 H H C4 OH H C5 OH CH2 OH 6 5 4 1 3 2 Anomeric carbon (most oxidized) For all non-anomeric carbons, -OH groups point down in Haworth projections if pointing right in Fischer projections 4 Conformation of Monosaccharides Pyranose sugars not planar molecules, prefer to be in either of the two chair conformations. Reducing Sugars • When in the uncyclized form, monosaccharides act as reducing agents. • Free carbonyl group from aldoses or ketoses can reduce Cu2+ and Ag+ ions to insoluble products Derivatives of Monosaccharides 5 Sugar Phosphates Deoxy Acids Amino Sugars 6 Sugar alcohols Monosaccharide structures you need to know 1) 2) 3) 4) 5) 6) Glucose Fructose Ribose Ribulose Galactose Glyceraldehyde 7