Expt. 2
advertisement

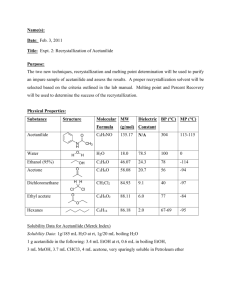
Chem 2LA3 Analgesic Drug Preparation Experiment 2. Analysis of an Analgesic Drug Preparation * adapted from J.W. Lehman, “Multiscale Operational Organic Chemistry”, Prentice-Hall (2002), pp. 28-39 * Techniques Gravity filtration [TCD-16] Extraction [TCD-18] Recrystallization [TCD-24] Melting point [TCD-33] Vacuum filtration [TCD-17] Evaporation [TCD-19] Heating [TCD-9] Weighing [TCD-4/5] Drying solids [TCD-22] Scenario. A client has submitted a sample of two commercial analgesic drug preparations called “Aspophen I” and “Aspophen II” to our laboratories for testing. The labels indicate that the two formulations each consist of the same three components, but in different relative amounts. Each tablet of Aspophen I is supposed to contain aspirin (200 mg), acetaminophen (250 mg) and sucrose (50 mg), while each tablet of Aspophen II is supposed to contain aspirin (300 mg), acetaminophen (150 mg), and sucrose (50 mg). Our client believes that both Aspophen I and II do indeed contain aspirin and sucrose, but suspects that the third component is not acetaminophen, but rather a cheap chemical relative of acetaminophen, either acetanilide or phenacetin. They further suspect that the amounts of each component listed on the labels are incorrect, and furthermore that the identity of the third component may be different in the two formulations. Both acetanilide and phenacetin are banned in Canada because of their toxicity, so if either of them are present in these formulations then they need to be removed from the market as soon as possible. OCOCH3 COOH NHCOCH3 NHCOCH3 NHCOCH3 HO HO OH aspirin (acetylsalicylic acid) H OH acetanilide HO H OH H O OCH2CH3 acetaminophen phenacetin sucrose H OH OH O CH2OH CH2OH Figure 1 You are to analyze one of the two drug preparations to determine the approximate percentages of aspirin, sucrose, and the unknown component it contains, and determine the identity of the unknown component. Experiment # 2 Page 1 of 7 Chem 2LA3 Analgesic Drug Preparation Suggested Procedure. The three components of the mixture should be separable by taking advantage of solubility differences and the different acid-base properties of the various possible structures. Sucrose, a disaccharide, is just common table sugar and is the only compound in the mixture that is soluble in water and insoluble in organic solvents. Since the four analgesic compounds are all soluble in organic solvents such as dichloromethane, we should be able to isolate the sucrose by gravity filtration, after first mixing the preparation with dichloromethane to dissolve the aspirin and the unknown component. The aspirin can then be isolated from the dichloromethane solution by extraction with an aqueous solution of sodium hydroxide. Aspirin is a carboxylic acid, so treating it with base will convert it to its sodium salt, which is soluble in water. The unknown component, if it is acetanilide or phenacetin, will remain behind in the dichloromethane. After separating the aqueous phase from the organic phase, the aspirin can be precipitated from the aqueous solution by acidifying it with hydrochloric acid, and then isolated by vacuum filtration. The unknown can then be isolated from the remaining dichloromethane solution by simply evaporating the solvent. The percentage composition of the mixture can be determined from the masses of the individual components, after drying them. Note that the actual composition may not be the same as that given in the scenario. Careful work is required to obtain accurate results; errors can arise from incomplete mixing with dichloromethane, incomplete extraction or precipitation of the aspirin, incomplete drying of the recovered components, losses in transferring substances from one container to another, or errors in weighing. The unknown component will be purified by recrystallization and then analyzed to establish its identity and degree of purity. Recrystallization is the most commonly used method for purification of solids; others include chromatography and sublimation, which you will experience in later experiments. Liquids are usually purified by distillation or chromatography. The solubility information given in the Table below indicates that while acetanilide and phenacetin are both insoluble in cold water, they are both relatively soluble in boiling water. This means that it should be possible to purify both compounds by recrystallization using water as the solvent. Table 1 Physical properties of acetanilide and phenacetin M.W. m.p. Acetanilide 135.2 o Solubility, cold water Solubility, hot water 114 C 0.54 5.0 Phenacetin 179.2 135 oC 0.076 1.22 a. In grams of solute per 100 mL solvent Based on the mass of unknown that you recover from the extraction procedure, you can estimate the volume of boiling water needed to dissolve it depending on whether it is acetanilide or phenacetin. For example, if the unknown is acetanilide and you recovered 1.50 g of the crude solid from the extraction, then the amount of boiling water needed to dissolve it is calculable from the solubility in boiling water (usually given in g / 100 mL) and the mass of the sample: Volume water needed = 1.50 g acetanilide x (100 mL water / 5.0 g acetanilide) = 30 mL Phenacetin is less soluble in boiling water than acetanilide, and will hence require more water to dissolve the same amount. You should begin the recrystallization process using the smaller volume of water (don’t add it all at once, and add more only if your compound doesn’t dissolve in that amount of water at its boiling point. Experiment # 2 Page 2 of 7 Chem 2LA3 Analgesic Drug Preparation Because the properties of both acetanilide and phenacetin are already reported in the chemical literature, we can identify the unknown and assess its purity by its melting point range. If your unknown is acetanilide then it should melt sharply near 114 oC, while if it is phenacetin it should do so near 135 oC. Pre-lab - Purpose of the experiment Brief outline of procedure to be used table of physical properties of aspirin, acetanilide, phenacetin, and sucrose (e.g. see Table above) table of physical properties of solvents to be used (M.W., density, boiling point) flow chart illustrating the extraction procedure to be followed (see Appendix A) Directions. The experiment will be completed over two lab periods, with all operations except melting point determinations done in the first period. This is to allow time for adequate drying of solids prior to determining their melting points. (a) Separation of sucrose. Accurately weigh about 3.00 g of Aspophen I or II on a creased piece of weighing paper and transfer it to a clean, dry 125-mL Erlenmeyer flask. Add 50 mL of dichloromethane to the flask. Stir the mixture thoroughly with a stirring rod to dissolve as much of the solid as possible, using the rod to break up any lumps or granules that may be present. Using a pre-weighed fluted filter paper, filter the solution by gravity into a small flask, saving the filtrate for the next step. It is crucial that the contents of the flask be poured quickly into the filter paper; if it is done slowly, the solvent will evaporate and cause the dissolved solid to crystallize in the filter paper and funnel. This will slow the filtration considerably, and lead (among other things) to poor recovery. Ask a TA for advice if you think your filtration is going too slowly. Rinse the original flask with about 10 mL of fresh dichloromethane, and use this solution to rinse the filter paper and funnel, collecting it in the filter flask along with the bulk of the filtrate. After repeating the rinsing procedure a second time, set the filter paper aside in a small beaker, taking care not to lose any of the sucrose, and reweigh it when it is completely dry. Record the mass of the sucrose in your laboratory notebook. After you have shown your sample of recovered sucrose to a TA, it should be placed in the jar labeled “Sucrose” that is located in the waste hood. (b) Separation of Aspirin. Transfer the filtrate to a separatory funnel and extract it with two separate 25-mL portions of aqueous 1 M sodium hydroxide. Because the density of dichloromethane is greater than that of water, the organic layer will be on the bottom, and you will thus have to transfer each layer to a different (labeled) container – return the dichloromethane layer to the separatory funnel before the second extraction. Combine the two aqueous extracts in the same container and save the dichloromethane layer, which should now contain only the unknown component, for the next step. Slowly add 10 mL of 6 M hydrochloric acid to the combined aqueous extracts while stirring with a glass rod. Test the pH of the solution with pH paper (to do this, use your stirring rod to transfer a drop of the solution to a strip of pH paper – do not dip the pH paper in the solution), and if necessary add more acid to bring the pH down to 2 or lower. Cool the mixture in an ice/water bath for at least 10 minutes, collect the aspirin by vacuum filtration, and wash it on the filter with cold water. Let the aspirin dry on the filter for a few minutes with the aspirator running, then dry it to constant mass. Weigh the aspirin and record its mass in your lab notebook. After you have Experiment # 2 Page 3 of 7 Chem 2LA3 Analgesic Drug Preparation shown your sample of recovered aspirin to a TA, it should be placed in the jar labeled “Aspirin” that is located in the waste hood. (c) Isolation of the Unknown Component. Dry the dichloromethane solution containing the unknown from (b) over anhydrous sodium sulfate, gravity-filter it into a clean, dry 100-mL roundbottom flask, and use the rotary evaporator to evaporate the solvent. Transfer the solid that remains to a pre-weighed vial and let it dry to constant mass. (d) Compositional Analysis. Calculate your percent recovery by dividing the sum of the masses of all components by the mass of Aspophen that you started with. Calculate the approximate percentage composition of Aspophen, based on the total mass of components recovered (these percentages should add up to 100%). (e) Purification of the Unknown Component. Recrystallize the unknown component by boiling it with just enough water to dissolve it completely, then letting it cool slowly to room temperature. Use an Erlenmeyer flask large enough to accommodate the maximum amount of recrystallization solvent that might be required. If necessary, induce crystallization by scratching the sides of the flask with a glass stirring rod (consult a TA for advice on how to do this, if necessary). Then cool the flask further in an ice/water bath to increase the yield of product. Collect the solid by vacuum filtration, washing it with a small amount of ice-cold water. Dry the product to constant mass, transfer it to a pre-weighed vial, and determine the amount recovered from the recrystallization. (f) Analysis & Identification of the Unknown Component. Grind a small amount of the purified, dry compound to a fine powder on a watch glass using a spatula or a flat-bottomed stirring rod. Measure the melting point range of the purified unknown, recording the temperature at which you see the first trace of liquid and the temperature at which the sample becomes completely liquid. Carry out at least two measurements with the sample. Turn in the remaining product to your TA. Waste Disposal. Place recovered dichloromethane in the “Halogenated Waste” container. Conclusions. These should begin with the statement… “Analysis of Aspophen I or II indicates it to consist of: - sucrose (x% by weight) acetylsalicylic acid (y% by weight) identity of unknown (z% by weight)” Indicate the percent recovery of material from the extraction procedure, and give your opinions on the most likely reasons for material loss. Also indicate the most likely sources of other errors in the compositional analysis. Experiment # 2 Page 4 of 7 Chem 2LA3 Analgesic Drug Preparation Appendix A. Planning and executing extraction procedures Planning and keeping track of an extraction procedure always takes some thought, and it’s easy to get things mixed up. Even seasoned veterans find it useful to follow two basic rules in planning and executing an extraction procedure: (1) organize yourself before beginning by drawing up a flow chart outlining the procedure to be followed; and (2) never throw anything away until you’re sure it is really waste. A flow chart illustrating the separation of the analgesic drug mixture of this experiment by solvent extraction should look something like this: ASA + acetanilide/phenacetin + sucrose (solid mixture) 1. dichloromethane 2. filter organic phase solid phase sucrose (solid) ASA + acetanilide/phenacetin in CH2Cl2 1M NaOH (aqueous) aq. phase (UPPER) org. phase (LOWER) acetanlide/phenacetin in CH2Cl2 OCOCH3 CO2-Na+ dry and evaporate sodium acetylsalicylate in water acetanilide or phenacetin (solid) 1. 6M HCl 2. filter acetylsalicylic acid (solid) Figure 2 Experiment # 2 Page 5 of 7 Chem 2LA3 Analgesic Drug Preparation Note that in this example, there is only one fraction for which rule (2) above would apply: the (aqueous) filtrate from the last step. Throw it away only once you’re satisfied that you’ve recovered more or less the expected amount of solid (ASA) in the filtration, and that the solid recovered is indeed what you expect it to be. In the meantime, keep it in a loosely stoppered, labeled Erlenmeyer flask in your fume hood. A somewhat more complicated example is given by the separation of a mixture of 4-chloroaniline (a solid amine; soluble in aq. acid & organic solvents), benzoic acid (a solid carboxylic acid; soluble in aq. base & organic solvents), and toluene (a neutral liquid hydrocarbon; soluble in organic solvents). This mixture is a viscous liquid at room temperature; none of the components are soluble in neutral water, and all of them are soluble in organic solvents. We again begin the procedure by taking up the mixture in an organic solvent, this time diethyl ether (d25C = 0.706 g/mL; bp = 34.6 oC). p-chloroaniline + benzoic acid + toluene in ether 2M HCl (aqueous) org. phase (UPPER) benzoic acid + toluene in ether Cl toluene in ether dry and distill toluene (liquid) NH3 Cl p-chloroanilinium chloride in water 5% NaHCO3 (aqueous) org. phase (UPPER) aq. phase aq. phase CO2-Na+ 1. 10% NaOH 2. filter p-chloroaniline (solid) sodium benzoate in water 1. 6M HCl 2. filter benzoic acid (solid) Figure 3 Note that in this example there are two fractions for which rule (2) above applies. Experiment # 2 Page 6 of 7 Chem 2LA3 Analgesic Drug Preparation Experiment # 2 Page 7 of 7