fall_LFU_extra1
advertisement

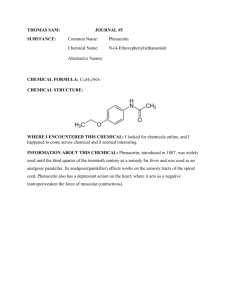
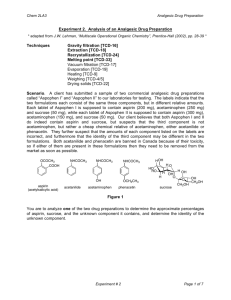
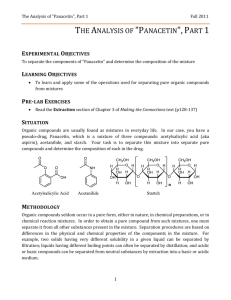
CHM 253L Organic Chemistry Laboratory I Laboratory Follow-Up Assignment #4 Due: Wednesday, October 6, 2004 by 5:00 pm 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Calculate your percent recovery by dividing the mass of each component by its initial mass that you were given at the beginning of the experiment. Do you think your numbers are reasonable? Why or why not? Comment on ALL POTENTIAL influences that may have decreased your yield. Explain by commenting on the efficiency of separation achieved in each step of the process (e.g. gravity filtration, extraction, precipitation, etc.). Report your unknown letter, experimental and literature melting points. Did you have acetanilide or phenacetin as your unknown? How did you determine its identity? What was the specific function of using sodium hydroxide in the separation of the common analgesic (aspirin)? Be sure to show chemical reactions to support your ideas. Draw a series of pictures to describe how the common analgesic (aspirin) moved from the organic layer to the aqueous layer during the extraction process. Even with two extractions with 25 mL each of NaOH (aq), do you think that ALL of the aspirin "moved" from the organic layer to the aqueous layer? Why or why not? Do you think there is a possibility that some of the unknown (either acetanilide or phenacetin) "moved" from the organic layer into the aqueous layer with the aspirin? Why or why not? Given your answers to #5 and #6, would you consider extraction to be a “PERFECT” method of separation? Explain. Describe and explain the possible effects on your results of the following experimental errors or variations. In each case specifying the component(s) whose percentage(s) would be too high or too low. a. After adding dichloromethane to your drug preparation, you didn’t stir the mixture long enough. b. During the NaOH extraction, you failed to mix the aqueous and organic layers thoroughly. c. You mistakenly extracted the dichloromethane solution with 1 M HCl rather than 1 M NaOH. d. You only performed a single extraction of the dichloromethane layer with 1 M NaOH. What problem would you encounter if the unknown component was replaced with acetaminophen rather than using acetanilide or phenacetin? Think: you have replaced your original aspirin/acetanilide/phenacetin mixture (in CH2Cl2) with an aspirin/acetaminophen mixture. The important thing to realize is that acetaminophen is a weaker acid than aspirin, but a stronger acid than either acetanilide or phenacetin. One of the main problems in synthetic drug chemistry is separating unwanted side products (like indole) from the intended product (5-OMeDMT). This is particularly a problem if you're in the bad habit of buying street-grade drugs. Based upon what you learned in this experiment, how would you go about separating a mixture of 5-methoxyDMT (free base) and indole? 11. Tell whether each of the following experimental errors will raise or lower the amount of product you recover, and why. a. You failed to dry the product completely. b. You used enough water to recrystallize phenacetin but your unknown was acetanilide. c. You did not extract all of the common analgesic from the dichloromethane solution during the NaOH extraction. Laboratory Follow-Up Assignment #9 Due: Friday, November 22, 2002 by 5:00 pm Be sure to employ LABELED STRUCTURES to illustrate critical relationships!!!! NOTE: You can complete #1-3 directly on the spectra 1. Provide a complete analysis for your 1D proton NMR spectrum including: (a) a drawing of the structure with all hydrogen atoms labeled (b) close-up views of split regions of the spectrum with integrals (c) close-up views of split regions with values for the coupling constants labeled 2. Provide peak assignments for each signal in your 1D 13C NMR (correlated with a drawing of the structure with all carbon atoms labeled). 3. Identify various functional groups present in your structure from the IR spectrum and the major information gathered from the GCMS data. 4. Provide peak assignments for each signal in your 13C DEPT spectrum correlated with the different categories of carbons (methyl, methylene, and methine). 5. Provide a complete analysis of your COSY spectrum: (a) Identify each cross peak and give it a label (letter or number) (b) Draw appropriate lines of the spectrum indicating which peak regions are coupled (c) Create a table in which you list: the cross peaks the identity of the coupled hydrogen atoms that yield the cross peak the relative size of the cross peak (small, medium, or large) the value of the coupling constant for the coupled hydrogen atoms indicate the type of coupling exhibited (vicinal, geminal, four-bond, five-bond, or W-coupling) 6. Provide a complete table of dihedral angles and coupling constants as determined by PCSpartan Plus. 7. Provide a short (1 page) discussion of how the data enabled you to determine the structure of your unknown terpenoid. Be sure to include how you assimilated the data to confirm the structure of the terpenoid -- pay particularly close attention to evaluation of the molecular modeling and 2-D NMR data as it pertains to making unequivocal assignments of the various hydrogen atom resonances.