Mathcad - Thermo Equation Sheet
advertisement

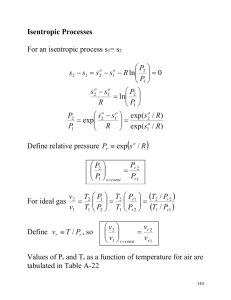
w := P⋅ v ⋅ ln ⌠ w := P d v ⌡ Closed System, Reversible, w := P( v 2 − v 1) P = Const. w := R( T2 − T1) Cp = Const. P1 P2 w := R⋅ T⋅ ln w := P = Const. I.G. Heat = Work 1st Law of Thermodynamics v2 v1 Closed System: n := 1 (P2⋅ v2 − P1⋅ v1) Closed System q − w := u 2 − u 1 Delta KE ~ 0 Delta PE ~ 0 T = Const. Reversible I.G. Pv^n = Const. 1 n≠1 1−n Comp. P X is Quality δQ := δW x := massvap masstotal Sat. 1 − x := massliq 0 masstotal 0 v v := v f + x⋅ v fg Saturated u := u f + x⋅ u fg Saturated h := u + Pv Always v ≡ v f @ T Compressed u ≡ u f @ T Compressed look up h in charts Superheated look up v in charts Superheated look up u in charts Superheated Steady Flow Systems (St. Fl.): ⌠ w := − v d P ⌡ w := n ⋅ Reversible St. Fl. ∆KE ~ 0 ∆PE ~ 0 1−n Q := q ⋅ m 2 St. Fl. − g ⋅ ( z − z ) Reversible 2 1 St. Fl. v2 Reversible v 1 Isothermal P1 n = 1 w := R⋅ T⋅ ln ⋅ P2 w := −v ⋅ ( P2 − P1) m := ρυA St. Fl. ρ := m := v = Const. P I.G. R⋅ T υA not an I.G. v w := −R⋅ ( T2 − T1) I.G. 1st Law of Thermodynamics i Heat Engines: n =1 qh = heat gained qH qL = heat rejected q L Thermal Efficiency ∑ Q+ St. Fl. q − w := h 2 − h 1 ∆KE ~ 0 ∆PE ~ 0 := TH i ∑ n =1 (υ i)2 mih i + + g ⋅ zi 2 St. Fl. For Pumps or Engines 1 ζth := 1 − qH W net TL qH TH T 0.5 W net := q H − q L qH q := T⋅ ( S2 − S1) (υ i)2 mih i + + g ⋅ zi = 2 TL qL Assume Carnot! ζth := 2 w := P⋅ v ⋅ ln ⋅ m = mass flow rate (Const.) ζth := 1 − 1 q's don't change form St. Fl. to Closed System υ − υ ⌠ 1 2 w := − v d P − 2 ⌡ Pv^n = Const. I.G. n = 0, P = Const. n = 1, T = Const. n = κ, S = Const. n to ∞, v = Const. St. Fl. (P2⋅ v2 − P1⋅ v1) W := m⋅ w Super qL 0 reversible isothermal Heat Pumps: 0 0.5 S 1 1 Heat Pumps: Coefficient of Performance α := β := qH α := W net qL β := W net qh = heat rejected qH qL = heat gained qL TH := For Pumps or Engines TL 1 qH α − β := 1 qH − qL qH W net := q H − q L T qL qH qH − qL qH − qL := 0.5 TH qL TH − TL 0 0 0.5 w := −v ⋅ ( P2 − P1) ( κ − 1) Other Equations: P2 := T1 P1 T2 P⋅ V := nR ( bar) T P⋅ V := mRT R := ρ := R( bar) I.G. (all 5) M P δh Cp := R⋅ T P < 10 MPa Pv := RT I.G. T > 2 Tcrit Cv := κ Pv^κ = Const. which implies: δu δT ⌠ h 2 − h 1 := Cp d T ⌡ ∆h := Cp ⋅ ∆T ⌠ u 2 − u 1 := Cv d T ⌡ Cp − Cv := R Cp := u(T) only Cp T2 v2 + R⋅ ln T1 v 1 C = Const. p T2 P2 I.G. ∆S := Cp ⋅ ln − R⋅ ln P T1 1 ( κ ⋅ R) κ−1 Pr1 := P2 P1 Cp (is not) = Const. I.G. adiabatic h1 = h2 absolutely not reversible R κ−1 Isentropic Cp is not Const. ∆S = 0 0 Throttling: h ~ hf (@ T) + vf (P - Psat) compressed or look in compressed tables 2 It's commonly an I.G. if Cp = Const., then Cv = Const. h := u + Pv Always Pr2 It's commonly an I.G. := κ Cv := ∆S := Cv⋅ ln P2 ∆S := S2 − S1 − R⋅ ln P1 h(T) only Cp = Const. Cv TdS := dh − vdP 0 h(T) only u(T) only Cv = Const. ∆u := Cv⋅ ∆T TdS := du + Pdv S = Const. Cp = Const. I.G. δT ⌠ reversible q := T d S ⌡ q := T⋅ ( S2 − S1) reversible isothermal ⌠ 1 P2 ∆S := Cp ⋅ d T − R⋅ ln T P1 ⌡ 1 S n n n n = 0, P = const. = 1, T = const. = R, S = const. -> ∞, v = const. 1 T P 0 S 0 v 1 Devices: Turbines Compressor 1 1 T T 0 0 0.5 0 1 0 0.5 S S Adiabatic Adiabatic γ tur := Steady Flow in Ideal case, Isentropic Wa γ comp := Steady Flow Ws 1 T No Graph T 0 0.5 0 1 0 S Adiabatic 3 kg 0.5 1 S γ pump := Steady Flow m Wa Nozzle 1 v := .001⋅ Ws W s := h 1 − h 2s I.G. Pump 0 1 2 Ws Adiabatic Wa Steady Flow W s := −v ⋅ ( P2 − P1) v := v f @T 3 γ noz := νa νs 2 Diffuser 1 T 0 0 0.5 1 S Adiabatic γ diff := Steady Flow ∆Pa ν1 > ν2 ∆Ps P2 > P1 2 2 P1 ν 1 P2 ν 2 + + g ⋅ z1 := ρ + 2 + g ⋅ z2 ρ 2 for ν is less than .5 moch 3 Part Cycle: Pr2 Pr1 := P2 P1 Isentropic Cp is not Const. ∆S = 0 ( k− 1) P2 P1 k T2 := T1⋅ Closed System q − w := ∆u Closed System P⋅ v := n ⋅ R⋅ T I.G. P2 I.G. P1 w := R⋅ T⋅ ln ⋅ USUF: (uniform state/ uniform flow) i = in the tank e = exit the tank 1 = initially in the tank 2 = final in tank Q + mi⋅ h i + ν 2 i 2 + g ⋅ zi := W + me⋅ h e + ν 2 e 2 + g ⋅ ze + m2⋅ u 2 + 4 ν 2 2 2 + g ⋅ z2 + m1⋅ u 1 + ν 2 1 2 + g ⋅ z1 Definitions: Isothermal: T = Const. Isentropic: S = Const. means (Reversible & Adiabatic) Adiabatic: q = 0 Reversible: Both the system and surroundings can be returned to their initial state Heat Engines: Thermo cycle with + net heat transfer and + net work Heat Pumps: Thermo cycle with - net heat transfer and - net work Steady Flow: No change @ a point with time Throttling: Adiabatic, h1 = h2 (for single inlet, single outlet only), & Absolutely not Reversible Pumps: Adiabatic Turbines: Adiabatic Compressors: Adiabatic Nozzles: Adiabatic Diffusers: Adiabatic Units Conversions: Charts: Description Air Critical Properties Critical Property Graphs H2O Propane R-22 I.G. Properties of Selected Gases Thermo Properties of selected Subs. I.G. Specific Heats of common Gases 5 SI English p. 840 p. 803 p. 899 p. 804 p. 832 p. 814 p. 842 p. 847 p. 838 p. 890 p. 851 p. 851 p. 852 p. 881 p. 864 p. 892 p. 897 p. 888 Properties of Various Ideal Gases SI Units Gas Chemical Formula Air Argon Butane Carbon Dioxide Carbon Monoxide Ethane Ethylene Helium Hydrogen Methane Neon Nitrogen Octane Oxygen Propane Steam Molecular Weight R( KJ ) Kg° K Cp( KJ ) Kg ° K Cv( KJ ) Kg ° K κ Ar C4 H10 28.97 39.948 58.124 .28700 .20813 .14304 1.0035 .5203 1.7164 .7165 .3122 1.5734 1.400 1.667 1.091 CO2 44.01 .18892 .8418 .6529 1.289 CO C2 H6 C2 H4 He H2 CH4 Ne N2 C8 H18 O2 C3 H8 H2 O 28.01 30.07 28.054 4.003 2.016 16.04 20.183 28.013 114.23 31.999 44.097 18.015 .29683 .27650 .29637 2.07703 4.12418 .51835 .41195 .29680 .07279 .25983 .18855 .46152 1.0413 1.7662 1.5482 5.1926 14.2091 2.2537 1.0299 1.0416 1.7113 .9216 1.6794 1.8723 .7445 1.4897 1.2518 3.1156 10.0819 1.7354 .6179 .7448 1.6385 .6618 1.4909 1.4108 1.400 1.186 1.237 1.667 1.409 1.299 1.667 1.400 1.044 1.393 1.126 1.327 English Units Gas Chemical Formula Air Argon Butane Carbon Dioxide Carbon Monoxide Ethane Ethylene Helium Hydrogen Methane Neon Nitrogen Octane Oxygen Propane Steam Molecular Weight R( ft ⋅ lbf ) lbm° R Cp( Btu ) lb m°R Cv( Btu ) lbm° R κ Ar C4 H10 28.97 39.94 58.124 53.34 38.68 26.58 .240 .1253 .415 .171 .0756 .381 1.400 1.667 1.09 CO2 44.01 35.10 .203 .158 1.285 CO C2 H6 C2 H4 He H2 CH4 Ne N2 C8 H18 O2 C3 H8 H2 O 28.01 30.07 28.054 4.003 2.016 16.04 20.183 28.016 114.22 32.000 44.097 18.016 55.16 51.38 55.07 386.0 766.4 96.35 76.55 55.15 13.53 48.28 35.04 85.76 .249 .427 .411 1.25 3.43 .532 .246 .248 .409 .219 .407 .445 .178 .361 .340 .753 2.44 .403 .1477 .177 .392 .157 .362 .335 1.399 1.183 1.208 1.667 1.404 1.32 1.667 1.400 1.044 1.395 1.124 1.329 SI: (Cp, C v , & κ are at 300 K), English: (Cp, C v , & κ are at 80 F) 6