Isentropic Processes
advertisement

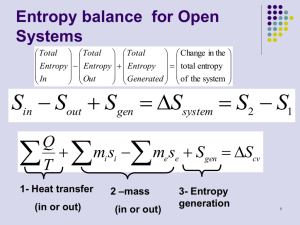
Isentropic Processes For an isentropic process s1= s2 P s 2 s1 s 2o s1o R ln 2 0 P1 P s 2o s1o ln 2 R P1 s 2o s1o exp(s 2o / R ) P2 exp o P1 R exp( s 1 / R) Define relative pressure Pr exps o / R Pr 2 P2 P 1 s const Pr 1 For ideal gas v2 T2 P1 T2 Pr1 T2 / Pr 2 v1 T1 P2 T1 Pr 2 T1 / Pr1 Define vr T / Pr , so v v2 r2 v1 s const vr 1 Values of Pr and Tr as a function of temperature for air are tabulated in Table A-22 144 Isentropic Process for ideal gas with constant cv and cP T2 v2 s 2 s1 cV ln R ln 0 T1 v1 note cV R /(k 1) 1 T v 0 R ln 2 ln 2 v1 k 1 T1 1 k 1 T v 0 ln 2 ln 2 T1 v1 1 k 1 T v 0 ln 2 2 T1 v1 Take exponential of both sides T exp(0) 1 2 T1 1 k 1 v 2 v1 T2 v1 s const T 1 c const v2 k 1 p but Pv v T2 P2 v2 substituting 2 2 1 P1v1 v2 T1 P1v1 k 1 145 k this yields P2 v 1 or P1v1k P2 v2k P1 cs const v2 const p Recall, for a polytropic compression or expansion process Pvn= const, for the special case of an isentropic process (adiabatic and reversible) n= k Combining the two equations yields 1 k v1 P2 T 2 v2 P1 T1 T2 P 2 T1 cs const P1 const 1 k 1 k 1 k p 146 Control volume entropy rate balance Similar approach to that used to derive conservation of energy m 2 m 1 CONTROL VOLUME m 3 Q j dSCV m i si m e se S gen j Tj i e dt Rate of Entropy change Rate of entropy transfer Rate of Entropy production If temperature in CV is not uniform Tj corresponds to the temperature at different points on the control surface where heat is transferred For steady-state, one inlet and one outlet, isothermal CV S gen 1 Q CV 0 sin sout m T m 147 Isentropic efficiencies of Turbines and Compressors Recall, for a turbine First Law (steady-state, neglecting KE and PE effects and heat losses) yields P1 Expansion (P2 < P1) W T WCV h1 h2 0 P2 m S gen An entropy balance yields s2 s1 0 m (Wout) For an actual turbine, irreversibilities are present, so accessible states are such that s2 > s1 1 T P1 2 P2 2s s The state labeled 2s on the T-s and h-s diagrams would be attained only in the limit of no irreversibilities, i.e., internally reversible expansion ( S gen 0 ) and thus s2 = s1 148 The maximum theoretical amount of turbine work output is obtained for an isentropic expansion WCV h1 h2 s m Since h1 - h2 < h1 - h2s the actual work produced is less than the ideal isentropic turbine produces The difference is gauged by the isentropic turbine efficiency defined by WCV / m h1 h2 t WCV / m s h1 h2 s Note, t < 1 149 Recall, for a compressor First Law (steady-state, neglecting KE and PE effects and heat losses) yields P1 C Compression (P2 > P1) WCV h1 h2 0 (Win) m W P2 An entropy balance yields s2 s1 S gen m 0 For an actual compressor irreversibilities are always present so s2 > s1 T 2 P2 2s P1 1 s The state labeled 2s on the T-s and h-s diagrams would be attained only in the limit of no irreversibilities, i.e., internally reversible compression where S gen 0 and thus s2 = s1 150 The minimum theoretical amount of compressor work required corresponds to isentropic compression WCV (h2 s h1 ) m Since h2 – h1 > h2s – h1 the actual work input is more than the ideal isentropic compressor requires The difference is gauged by the isentropic compressor efficiency defined by c WCV / m s WCV / m h1 h2 s h1 h2 Note, c < 1 151 Internally Reversible Steady-State Flow Work For a single inlet and exit (1-inlet, 2-exit) CV at steadystate neglecting KE and PE effects conservation of energy V12 V22 W CV Q CV g z1 z 2 h1 h2 m m 2 For an internally reversible process Q / m Tds WCV 12 Tds h1 h2 m Recall: Tds dh vdp 12 Tds h2 h1 12 vdp WCV h2 h1 12 vdP h1 h2 m For pumps, turbines, compressors when KE= PE= 0 W CV 12 vdP m int rev Pumps and compressors dP > 0 work done on system Turbines dP < 0 work done by system 152 Total Heat Transferred Total Work Liquids – liquid are incompressible, so v1=v2= v 2 W CV vdP v( P2 P1 ) 1 m int rev Gases - when each unit of gas through the CV undergoes a polytropic process Pvn= const 1 2 dP WCV n 2 vdP (const) 1 1 m 1 int rev Pn For the special case of an ideal gas where Pv = RT nRT1 T2 WCV 1 n 1 T1 m int rev n 1, 153 recall, for polytropic process W CV m int rev T2 P2 T1 P1 n 1 n n 1 nRT1 P2 n 1 n 1 P1 so n 1 Recall: If the process is internally reversible and adiabatic (isentropic) for constant cp and cv Pvk= const Substitute n= k in above equations to get work per unit mass for isentropic process (implies k const f (T ) ) For the case of n=1: P1v1 = P2v2 T1=T2 (isothermal) vdP gives: W CV RT ln P2 P1 m int rev n=1 154