Ch1a Supplemental Materials Extra Practice with Lewis Dot and
advertisement

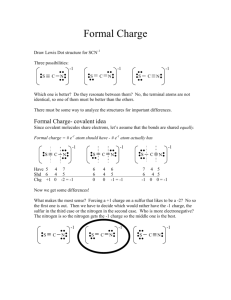
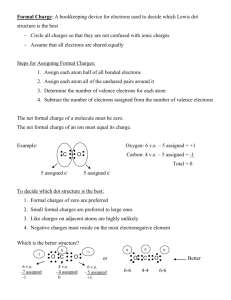
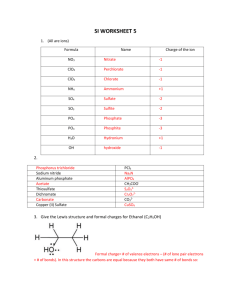
Ch1a Extra Practice with Lewis Dot and Resonance Structures Supplemental Materials Draw Lewis structures for the molecules below. Draw resonance structures where appropriate and label any formal charges. Rank resonance structures in order of importance to overall structure. 1.) [SCN]- 2.) [HC2O4]- 3.) SNF3 4.) [S2CO]2- 5.) [IO3]- 6.) [HCO3]- 7.) PF3 8.) [SO4]2- 9.) [C3H5]+ 10.) [S3N3]- (cyclic, every other atom is S) 11.) [ClO4]- (C is central) Page 1 of 1 Worksheet for Lewis Structures, Resonance Structures, and Formal Charge Solutions S 1.) N C C S N There are two things to consider here. The structure on the left gives N three bonds which is good, the structure on the right puts the formal negative charge on the more electronegative element. My guess is that the left structure is more important because N really likes to have 3 bonds. 2.) O O O H C C O These are equally important. N F 3.) S F F O O O H C C O 4.) O S C O S S C O S C S The right two structures are equally important and probably more important than the left structure (negative charge on more electronegative atom). 5.) O I O O I I O O O O I I O O O O O O O O O O I O The top three are equally important. The first two on the bottom are of equal importance. I think the bottom right two will be most important (fewer formal charges), then maybe S the bottom left structure (maximize bonding), then the top three structures. The order of importance isn’t entirely clear between the last two groups. (Argument between maximize bonding and put negative charge on the more electronegative atom.) O 6.) O H C O These two structures are equally important. F 7.) 8.) P F F O O H C O O O O O S S O O O O O O S O S S O O O S O O O O S O O O O S 2- O O O O O O O O O O S O O O O O These are pretty much in order of what I would expect the importance to be. O S O O 9.) H H C C C C C H H H H H H H C H These are of equal importance. No choice but to disobey the octet rule for one carbon. 10.) S S S N N N S S S N N N N S S S N N S S S N N N N N N S S S S S S N N N The three structures in the bottom row are equivalent and may be more important than the top three (which are equivalent with each other) due to fewer formal charges. 11.) O O Cl O O O Cl O O O O O Cl O O O O Cl O O These four are equally important. You might also want to draw structures where two oxygen atoms have negative charges and the chlorine has a positive charge. Those would be OK, but would be less important than this set because with this first set we don’t need to put a formal charge on more than one atom or to put a positive charge on an electronegative atom.