Synthesizing Organic Compounds
advertisement

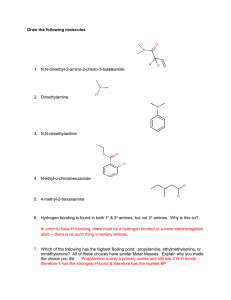
1.9 (a) There are many reasons why chemists create new organic substances. They may be synthesized as part of research or to demonstrate a new type of reaction. Others are synthesized if a compound is needed with specific chemical and physical properties. Large amounts of some synthetic compounds are routinely produced industrially (Figure 1). Chemical engineers can design and create synthetic replicas of naturally produced compounds by adding or removing key functional groups to or from available molecules. For example, there is a large market demand for caffeine as a stimulant for use in non-coffee beverages such as soft drinks and in solid foods. Caffeine is a cyclic compound containing nitrogen, and is extracted from coffee beans and tea leaves. To meet the large demand, a compound called theobromine is obtained from cocoa fruits and modified chemically to produce caffeine; the structures differ by the presence of a single methyl group, which is added to the theobromine. In recent years, however, the popularity of decaffeinated coffee has resulted in the increased availability of natural caffeine, removed from coffee in the decaffeination process. O C OH O C O H C H Synthesizing Organic Compounds H O ASA H (b) H H N H N O C O H N C C N O CH3 C C CH3 N C H N CH3 theobromine O N C C N CH3 C C N C H N CH3 caffeine urea Figure 1 (a) More than ten million kilograms of Aspirin, or acetylsalicylic acid (ASA), are produced in North America annually. Because of this compound’s ability to reduce pain and inflammation, it is the most widely used medicine in our society. (b) Urea, the organic compound first synthesized by Wöhler in 1828, is produced in even larger quantities than ASA. Urea is used primarily as plant fertilizer and as an animal feed additive. 80 Chapter 1 Throughout our study of organic families, we have focused on the transformation of functional groups. Let us take our knowledge of these reactions and devise a method of preparing a more complex molecule from simpler ones. Let’s say that we have been assigned the task of preparing an ester with a pineapple flavour, to be used in candies. We have available a number of short-chain alkanes and alkenes as starting materials. What substance will we make, which starting materials should we use, and what steps should we take in the synthesis? Pineapple Flavouring: Ethyl Butanoate When planning a synthesis pathway for any substance, we have a couple of options. One approach is to look at the available compounds and examine the transformations they can undergo. For example, what products can we obtain from short-chain alkanes and alkenes? We can then look at the possible products of these transformations and proceed further. Because there are many possible reactions and products, however, this approach presents several pathways to consider, many of which are ultimately unproductive. Another approach is to start with the compound we want to prepare—ethyl butanoate—and think “backwards.” Ethyl butanoate is, like all synthetic esters, derived from an acid and an alcohol. Having identified the acid and the alcohol, we then identify a reaction that would produce each of them from our choice of short-chain alkanes and alkenes. This approach limits the possible reactions and is a more efficient method of selecting a synthesis pathway. NEL Section 1.9 O CH3CH2CH2C O CH2CH3 ethyl butanoate Let us apply the “backwards” approach to the synthesis of ethyl butanoate, the ester with a pineapple flavour. As the name and structure show, this ester is formed from a reaction between ethanol and butanoic acid. The steps in our multi-step procedure are shown in the sample problem below. Of course, there is more than one possible synthesis pathway for any product; when selecting a procedure, industrial chemists always pay attention to the availability and cost of starting materials, as well as safety and environmental concerns. Designing Synthesis Pathways SAMPLE problem Write a series of equations to illustrate the synthesis of ethyl butanoate from an alkene and an alcohol from simpler molecules. First, plan a pathway for the production of ethyl butanoate. To make the “ethyl” portion of the ester, we will need to synthesize the necessary alcohol, ethanol. Ethanol can be prepared by the addition reaction of water to ethene, a hydration reaction. We will select ethene and water as the starting materials for this alcohol. Next, we will need to prepare butanoic acid. Recall that acids can be prepared from the controlled oxidation of aldehydes, which, in turn, are prepared from the controlled oxidation of alcohols. We need to determine which alcohol to use for this sequence. 1-butanol will produce butanal, which will produce butanoic acid. However, being a primary alcohol, 1-butanol is difficult to prepare from the reactions we have studied so far. The addition reaction of H2O to either 1-butene or 2-butene would produce predominantly 2-butanol, as predicted by Markovnikov’s rule. CH2 CHCH2CH3 H2O H2SO4 CH3CHCH2CH3 OH CH2CH CHCH3 H2O H2SO4 CH3CHCH2CH3 OH Other reagents and reactions are available for preparing primary alcohols, as you may learn in future chemistry courses. For this synthesis, we will use 1-butanol as our starting material to prepare butanoic acid. Our pathway is now complete. ethyl butanoate (an ester) ↑ ↑ ethanol (alcohol) ↑ ethene (alkene) water butanoic acid (carboxylic acid) ↑ butanal (aldehyde) (O) ↑ 1-butanol (alcohol) (O) The equations are 1. CH3CH2CH2CH2OH (O) → CH3CH2CH2CHO H2O 1-butanol butanal 2. CH3CH2CH2CHO (O) → CH3CH2CH2COOH butanal NEL butanoic acid Organic Compounds 81 3. CH2CH2 H2O → CH3CH2OH ethene ethanol 4. CH3CH2CH2COOH CH3CH2OH → CH3CH2CH2COOCH2CH3 butanoic acid ethanol ethyl butanoate Example Write a series of equations for the synthesis of propanone, starting with an alkene. Solution 1. CH3CH ACTIVITY 1.9.1 Building Molecular Models to Illustrate Reactions (p. 90) What do molecules really look like, and how does one substance turn into another? This activity helps you visualize, using molecular models, how reactions happen. CH2 H2O propene CH3CH(OH)CH3 2-propanol 2. O CH3CH(OH)CH3 (O) 2-propanol CH3CCH3 propanone Practice Understanding Concepts 1. A food additive that is named as a single ingredient is often a mixture of many ACTIVITY 1.9.2 Preparation of an Ester— Aspirin (p. 90) The reaction between salicylic acid and acetic anhydride produces a well-known pharmaceutical product. compounds. For example, a typical “artificial strawberry flavour” found in a milkshake may contain up to 50 different compounds. A few of these are listed below. For each, write a structural formula and an equation or a series of equations for a method of synthesis from the suggested reactants. (a) 2-pentyl butanoate from pentene and butanal (b) phenyl 2-methylpropanoate from phenol and an appropriate alcohol (c) 4-heptanone from an alcohol (d) ethyl ethanoate from ethene Section 1.9 Questions Understanding Concepts 1. For each product, write a structural formula and an equa- tion or a series of equations for a method of synthesis from other compounds. (a) pentyl ethanoate from ethene and an alcohol (b) phenyl ethanoate from an alkene and an alcohol (c) 3-octanone from a simpler compound (d) methyl benzoate from two alcohols (e) sodium salt of butanoic acid from an ester (f) trimethylamine from ammonia and alkanes (g) N-ethylethanamide from an alkane and ammonia 2. The smell of freshly cut grass can be simulated by the addi- tion of hexanal to substances such as air-fresheners. Describe how hexanal can be made from an alcohol. 82 Chapter 1 Applying Inquiry Skills 3. In artificial apple flavour, the main ingredient is ethyl 2-methylbutanoate. (a) Draw a flow chart to show the synthesis of this compound, starting with simpler compounds. (b) Describe the steps in the procedure for this synthesis. Include experimental conditions and safety precautions in the handling and disposal of materials. Making Connections 4. The flavour of almonds is obtained from phenyl methanal, also called benzaldehyde. “Natural” almond flavour is extracted from the pit of peaches and apricots, and may contains traces of the poison hydrogen cyanide. (a) Write a structural diagram for benzaldehyde. (b) Write equations for a series of reactions in the synthesis of benzaldehyde from an ester. NEL Section 1.9 (c) Research and describe the toxicity of the reactants listed in (b), and compare the toxicity to that of hydrogen cyanide. In your opinion, do your findings substantiate consumer perception that “natural” products are always healthier than their “artificial” counterparts? GO 6. If you were told that a neighbour was starting a new career in “organic products,” why might there be some confusion about what the new job involves? 7. Look at the product labels on foods, pharmaceuticals, and dietary supplements in a supermarket or drugstore. In particular, look for the terms “organic,” “natural,” and “chemical.” What is the purpose of using these terms on the packaging? Do they always mean the same thing? Do they have a positive or negative connotation? Write a short opinion piece on the use of these three terms, and what these terms mean to consumers. www.science.nelson.com 5. Quinine is a fairly effective treatment for malaria, a mainly tropical disease that kills or incapacitates millions of people every year. Quinine has a bitter taste and was mixed with lemon or lime to make it palatable; it was later included as a component of a beverage called tonic water. (a) Tonic water contains approximately 20 mg quinine per 375-mL can; the dosage required for the treatment of malaria is approximately six 300-mg tablets per day. In your opinion, would drinking tonic water be an effective approach to the treatment of malaria? Explain. (b) Refer to the structural diagram of quinine (Figure 2), and explain why quinine is soluble in water. (c) Research the effectiveness of quinine as an antimalarial agent from the early 1800s to the present. GO SUMMARY CH=CH2 N HO H CH2OH N Figure 2 Structure of quinine www.science.nelson.com Flow Chart of Organic Reactions alkenes addition + H2 + HX substitution alkanes + X2 alkyl halides + NH3 amines + H2O + H2O alcohols 1º alcohol (O)* aldehydes 2º alcohol (O)* ketones dehydration (H2SO4) ethers (O)* *(O) indicates controlled oxidation with KMnO4 or Cr2O72–, in H2SO4 carboxylic acids amine alcohol condensation amides NEL esters Organic Compounds 83