Draw the following molecules N,N-dimethyl-2-amino-2-chloro
advertisement
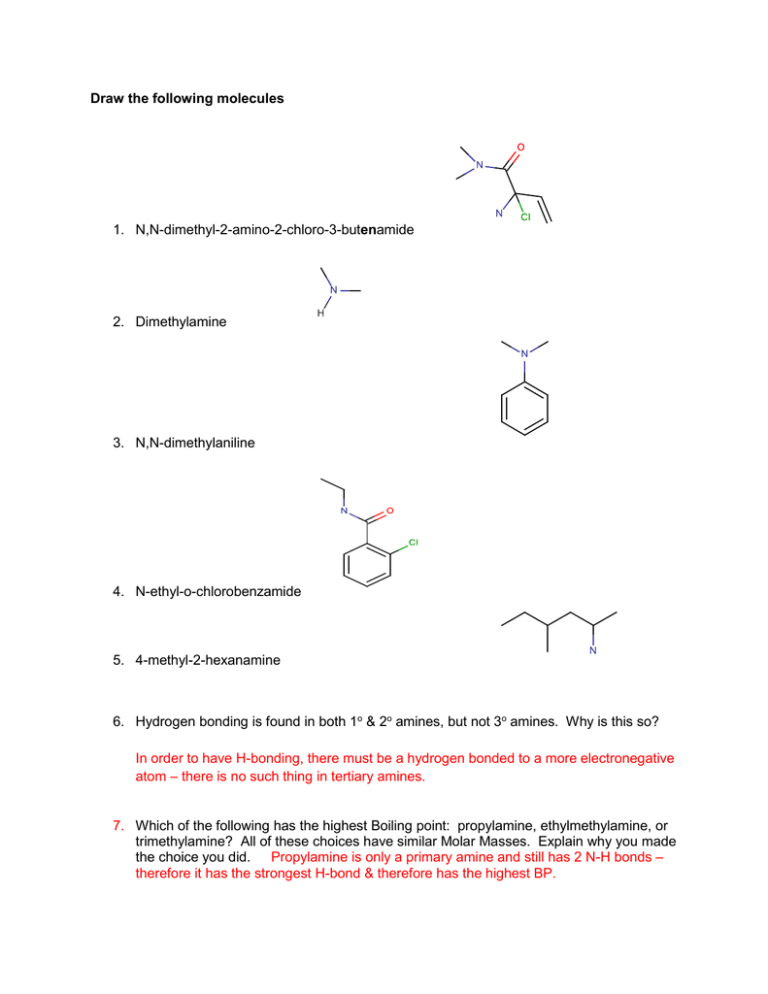
Draw the following molecules 1. N,N-dimethyl-2-amino-2-chloro-3-butenamide 2. Dimethylamine 3. N,N-dimethylaniline 4. N-ethyl-o-chlorobenzamide 5. 4-methyl-2-hexanamine 6. Hydrogen bonding is found in both 1o & 2o amines, but not 3o amines. Why is this so? In order to have H-bonding, there must be a hydrogen bonded to a more electronegative atom – there is no such thing in tertiary amines. 7. Which of the following has the highest Boiling point: propylamine, ethylmethylamine, or trimethylamine? All of these choices have similar Molar Masses. Explain why you made the choice you did. Propylamine is only a primary amine and still has 2 N-H bonds – therefore it has the strongest H-bond & therefore has the highest BP. Name the correct IUPAC name for the following structures: 1) pentanamide 2) N,N-dimethylmethanamide 3) ethyldiphenylamine or N-ethyl-N-phenylaniline 4) N-methyl-4-chloro-2-hexanamine 5) 4-amino-5-methoxyheptanamide 6) What 2 reactants would I need to make butanamide? Ammonia, butanoic acid Draw the following molecules 8. ethyl propionate (ethyl propanoate) 9. 3-bromo-4-methylpentanoic acid 10. o-methylbenzoic acid 4. butan-2-yl 3-cyclopropyl-5-oxooctanoate 5. m-methoxybenzoyl chloride Show the complete reaction for the formation of ethyl butanoate (pineapple smell); include the name & structural formulas of all species. water + ethanol + butanoic acid H+, heat water + + ethyl butanoate Write the correct IUPAC or Traditional name for the following: 7) 8) ethyl 3,6-dimethyloctanoate o-methoxybenzoic acid 9) phenyl propanoate (phenyl propionate) 10) butan-2-yl 3-chloro-4-methoxy-5-oxohexanoate 11) 2-hydroxy-4-hexenoic acid 12) What alcohol & acid would be needed to make pentyl ethanoate (banana smell)? a. Alcohol - pentanol b. Acid – ethanoic acid