Review Questions - Gordon State College
advertisement

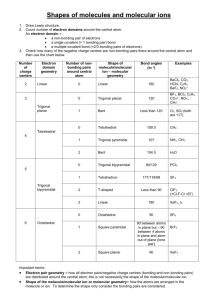
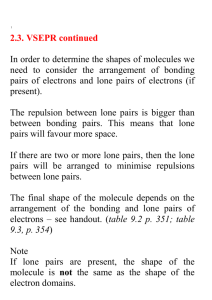
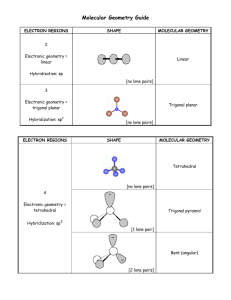
Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Review Questions 10.1 J The properties of molecules are directly related to their shape. The sensation of taste, immune response, the sense of smell, and many types of drug action all depend on shape-specific interactions between molecules and proteins. According to VSEPR theory, the repulsion between electron groups on interior atoms of a molecule determines the geometry of the molecule. The five basic electron geometries are (1) Linear, which has two electron groups. (2) Trigonal planar, which has three electron groups. (3) Tetrahedral, which has four electron groups. (4) Trigonal bipyramid, which has five electron groups. (5) Octahedral, which has six electron groups. An electron group is defined as a lone pair of electrons, a single bond, a multiple bond, or even a single electron. H—C—H ijj^^jl (a) Linear geometry \ \ (b) Trigonal planar geometry I 109.5= Tetrahedral geometry I Equatorial chlorine Axial chlorine "P—Cl: \ Trigonal bipyramidal geometry 1 369 I Octahedral geometry I 370 Chapter 10 Chemical Bonding II The electron geometry is the geometrical arrangement of the electron groups around the central atom. The molecular geometry is the geometrical arrangement of the atoms around the central atom. The electron geometry and the molecular geometry are the same when every electron group bonds two atoms together. The presence of unbonded lone-pair electrons gives a different molecular geometry and electron geometry. (a) Four electron groups give tetrahedral electron geometry, while three bonding groups and one lone pair give a trigonal pyramidal molecular geometry. (b) Four electron groups give a tetrahedral electron geometry, while two bonding groups and two lone pairs give a bent molecular geometry. (c) Five electron groups give a trigonal bipyramidal electron geometry, while four bonding groups and one lone pair give a seesaw molecular geometry. (d) Five electron groups give a trigonal bipyramidal electron geometry, while three bonding groups and two lone pairs give a T-shaped molecular geometry. (e) Five electron groups gives a trigonal bipyramidal electron geometry, while two bonding groups and three lone pair give a linear geometry. (f) Six electron groups give an octahedral electron geometry, while five bonding groups and one lone pair give a square pyramidal molecular geometry. (g) Six electron groups give an octahedral electron geometry, while four bonding groups and two lone pairs gives a square planar molecular geometry. Larger molecules may have two or more interior atoms. When predicting the shapes of these molecules, determine the geometry about each interior atom and use these geometries to determine the entire threedimensional shape of the molecules. To determine if a molecule is polar, do the following: 1. Draw the Lewis structure for the molecule and determine the molecular geometry. 2. Determine whether the molecule contains polar bonds. 3. Determine whether the polar bonds add together to form a net dipole moment. Polarity is important because polar and nonpolar molecules have different properties. Polar molecules interact strongly with other polar molecules, but do not interact with nonpolar molecules, and vice versa. 10.8 According to valence bond theory a chemical bond results from the overlap of two half-filled orbitals with spin-pairing of the two valence electrons. 10.9 According to valence bond theory, the shape of the molecule is determined by the geometry of the overlapping orbitals. 10.10 In valence bond theory, the interaction energy is usually negative (or stabilizing) when the interacting atomic orbitals contain a total of two electrons that can spin-pair. 10.11 Hybridization is a mathematical procedure in which the standard atomic orbitals are combined to form new atomic orbitals called hybrid orbitals. Hybrid orbitals are still localized on individual atoms, but they have different shapes and energies from those of standard atomic orbitals. They are necessary in valence bond theory because they correspond more closely to the actual distribution of electrons in chemicallybonded atoms. 10.12 Hybrid orbitals minimize the energy of the molecule by maximizing the orbital overlap in a bond. Chapter 10 Chemical Bonding II 373 10.29 Nonbonding orbitals are atomic orbitals not involved in a bond and will remain localized on the atom. 10.30 In Lewis theory, a chemical bond is the transfer or sharing of electrons represented as dots. Lewis theory allows us to predict the combination of atoms that form stable molecules, and the general shape of a molecule. Lewis theory is a quick way to predict the stability and shapes of molecules based on the number of valence electrons. However, it does not deal at all with how the bonds that we make are formed. Valence bond theory is a more advanced bonding theory that treats electrons in a quantum-mechanical manner. A quantitative approach is extremely complicated but a qualitative approach allows an understanding of how the bonds are formed. In valence bond theory, electrons reside in quantum-mechanical orbitals localized on individual atoms. When two atoms approach each other, the electrons and nucleus of one atom interact with the electron and nucleus of the other atom. If the energy of the system is lowered, a chemical bond forms. So, valence bond theory portrays a chemical bond as the overlap of two half-filled atomic orbitals. The shape of the molecule can be predicted from the geometry of the overlapping orbitals. Also, valence bond theory explains the rigidity of the double bond. However, valence bond theory falls short in explaining certain phenomenon such as magnetism and certain bond properties. Valence bond theory treats the electrons as if they reside in the quantum-mechanical orbitals that we calculate for an atom. This is an oversimplification that is partially compensated for by introducing the concept of hybridization. An even more complex quantum-mechanical model is molecular orbital theory. In molecular orbital theory, a chemical bond occurs when the electrons in the atoms can lower their energy by occupying the molecular orbitals of the resultant molecule. The chemical bonds in MO theory are not localized between atoms, but spread throughout the entire molecule. Molecular orbital theory uses trial functions to solve the Schrodinger equation for the molecules. In order to determine how well the trial function works, you calculate the energy, trying to minimize the energy. However, no matter how "good" your guess, you can never do better than nature at minimizing energy. These minimum-energy calculations for orbitals must be done by computer. All three of these models have strengths and weaknesses, none is "correct." What information you need, depends on which approach you use. Problems by Topic VSEPR Theory and Molecular Geometry / • 10.31 Four electron groups: A trigonal pyramidal molecular geometry has three bonding groups and one lone pair of electrons, so there are four electron pairs on atom A. 10.32 ~N. Three electron groups: A trigonal planar molecular geometry has three bonding groups and no lone pairs of electrons so there are three electron pairs on atom A. 1 10.33 I (a) 4 total electron groups, 4 bonding groups, 0 lone pairs A tetrahedral molecular geometry has four bonding groups and no lone pairs. So, there are four total electron groups, four bonding groups, and pairs. (b) 5 total electron groups, 3 bonding groups, 2 lone pairs A T-shaped molecular geometry has three bonding groups and two lone pairs. So, there are five total electron groups, three bonding groups, and two lone pairs. (c) 6 total electron groups, 5 bonding groups, 1 lone pairs A square pyramidal molecular geometry has five bonding groups and one lone pair. So, there are six total electron groups, five bonding groups, and one lone pairs. (a) 6 total electron groups, 6 bonding groups, 0 lone pairs An octahedral molecular geometry has six bonding groups and no lone pairs. So, there are six total electron groups, six bonding groups, and no lone pairs. (b) 6 electron groups, 4 bonding groups, 2 lone pairs A square planar molecular geometry has four bonding groups and two lone pairs. So, there are six total electron groups, four bonding groups, and two lone pairs. / V / 10.34 374 Chapter 10 Chemical Bonding II (c) 5 electron groups, 4 bonding groups, 1 lone pair A seesaw molecular geometry has four bonding groups and one lone pair. So, there are five total electron groups, four bonding groups, and one lone pair. PF3: Electron geometry-tetrahedral; molecular geometry-trigonal pyramidal; bond angle = 109.5° Because of the lone pair, the bond angle will be less than 109.5°. Draw a Lewis structure for the molecule: PF3 has 26 valence electrons. • c • • r • -p •• -F •• Determine the total number of electron groups around the central atom: There are four electron groups on P. Determine the number of bonding groups and the number of lone pairs around the central atom: There are three bonding groups and one lone pair. Use Table 10.1 to determine the electron geometry, molecular geometry, and bond angles: Four electron groups give a tetrahedral electron geometry; three bonding groups and one lone pair give a trigonal pyramidal molecular geometry; the idealized bond angles for tetrahedral geometry are 109.5°. The lone pair will make the bond angle less than idealized. (b) SBr2: Electron geometry-tetrahedral; molecular geometry-bent; bond angle = 109.5° Because of the lone pairs, the bond angle will be less than 109.5°. Draw a Lewis structure for the molecule: has 20 valence electrons. Determine the total number of electron groups around the central atom: There are four electron groups on S. Determine the number of bonding groups and the number of lone pairs around the central atom: There are two bonding groups and two lone pairs. Use Table 10.1 to determine the electron geometry, molecular geometry, and bond angles: Four electron groups give a tetrahedral electron geometry; two bonding groups and two lone pair give a bent molecular geometry; the idealized bond angles for tetrahedral geometry are 109.5°. The lone pairs will make the bond angle less than idealized. (0 CHC13: Electron geometry-tetrahedral; molecular geometry-tetrahedral; bond angle = 109.5° Because there are no lone pairs, the bond angle will be 109.5°. Draw a Lewis structure for the molecule: CHC13 has 26 valence electrons. •• Cl- /~i Cl •• : ci: Determine the total number of electron groups around the central atom: There are four electron groups on C. Determine the number of bonding groups and the number of lone pairs around the central atom: There are four bonding groups and no lone pairs. Chapter 10 Chemical Bonding II 375 Use Table 10.1 to determine the electron geometry, molecular geometry, and bond angles: Four electron groups give a tetrahedral electron geometry; four bonding groups and no lone pairs give a tetrahedral molecular geometry; the idealized bond angles for tetrahedral geometry are 109.5°; however, because the attached atoms have different electronegativities the bond angles are less than idealized. (d) CS2: Electron geometry-linear; molecular geometry-linear; bond angle = 180° Because there are no lone pairs, the bond angle will be 180°. Draw a Lewis structure for the molecule: C$2 has 16 valence electrons. Determine the total number of electron groups around the central atom: There are two electron groups on C. Determine the number of bonding groups and the number of lone pairs around the central atom: There are two bonding groups and no lone pairs. Use Table 10.1 to determine the electron geometry, molecular geometry, and bond angles: Two electron groups give a linear geometry; two bonding groups and no lone pairs give a linear molecular geometry; the idealized bond angle is 180°. The molecule will not deviate from this. 10.36 (a) CF4: Electron geometry-tetrahedral; molecular geometry-tetrahedral; bond angle = 109.5° Draw a Lewis structure for the molecule: has 32 valence electrons. Determine the total number of electron groups around the central atom: There are four electron groups on C. Determine the number of bonding groups and the number of lone pairs around the central atom: There are four bonding groups and no lone pairs. Use Table 10.1 to determine the electron geometry, molecular geometry, and bond angles: Four electron groups give a tetrahedral electron geometry; four bonding groups and no lone pairs give a tetrahedral molecular geometry; idealized tetrahedral bond angles for tetrahedral geometry are 109.5°. (b) NFj. Electron geometry-tetrahedral; molecular geometry-trigonal pyramidal; bond angle = 109.5° Because of the lone pair, the bond angle will be less than 109.5°. Draw a Lewis structure for the molecule: NF3 has 26 valence electrons. N Determine the total number of electron groups around the central atom: There are four electron groups on N. Determine the number of bonding groups and the number of lone pairs around the central atom: There are three bonding groups and one lone pair. Use Table 10.1 to determine the electron geometry, molecular geometry, and bond angles: Chapter 10 Chemical Bonding II 10.38 377 ClOa will have the smaller bond angle because lone pair-bonding pair repulsions are greater than bonding pair-bonding pair repulsions. Draw the Lewis structures for both structures: has 26 valence electrons. C1O4 ~ has 32 valence electrons. o •• •• •• oii •• There are three bonding groups and There are four bonding groups and one lone pair. no lone pairs. Both have four electron groups, but the lone pair in QO^ ~ will cause the bond angle to be smaller because of the lone pair-bonding pair repulsions. S?4 Draw a Lewis structure for the molecule: SF4 has 34 valence electrons. •• :F : .:F—•• s — F".• • • F • r . Determine the total number of electron groups around the central atom: There are five electron groups on S. Determine the number of bonding groups and the number of lone pairs around the central atom: There are four bonding groups and one lone pair. Use Table 10.1 to determine the electron geometry and molecular geometry: The electron geometry is trigonal bipyramidal so the molecular geometry is seesaw. Sketch the molecule: l\ (b) C1F3 Draw a Lewis structure for the molecule: C1F3 has 28 valence electrons. •• :F : : F ci F • Determine the total number of electron groups around the central atom: There are five electron groups on CI. 378 Chapter 10 Chemical Bonding II Determine the number of bonding groups and the number of lone pairs around the central atom: There are three bonding groups and two lone pairs. Use Table 10.1 to determine the electron geometry and molecular geometry: The electron geometry is trigonal bipyramidal so the molecular geometry is T-shaped. Sketch the molecule: -Cl (c) IF2 Draw a Lewis structure for the ion: IF-> ~ has 22 valence electrons. :F •• i -F •• Determine the total number of electron groups around the central atom: There are five electron groups on I. Determine the number of bonding groups and the number of lone pairs around the central atom: There are two bonding groups and three lone pairs. Use Table 10.1 to determine the electron geometry and molecular geometry: The electron geometry is trigonal bipyramidal so the molecular geometry is linear. Sketch the ion: [F (d) IBr4 I Draw a Lewis structure for the ion: IBr4 " has 36 valence electrons. Br« •• Br- • Br : Determine the total number of electron groups around the central atom: There are six electron groups on I. Determine the number of bonding groups and the number of lone pairs around the central atom: There are four bonding groups and two lone pairs. Use Table 10.1 to determine the electron geometry and molecular geometry: The electron geometry is octahedral so the molecular geometry is square planar. Sketch the ion: Br Br Br Br Chapter 10 Chemical Bonding II (a) N2 Draw the Lewis structure: Atom Number of Electron Groups LeftN Right N 2 2 Number of Lone Pairs 1 1 Molecular Geometry Linear Linear Sketch the molecule: N==N (b) Draw the Lewis structure: N N •H Atom Number of Electron Groups LeftN Right N 3 3 Number of Lone Pairs 1 1 Molecular Geometry Bent Bent Sketch the molecule: / \ N H (c) N2H4 Draw the Lewis structure: •• •• Atom Number of Electron Groups Number of Lone Pairs Molecular Geometry LeftN 4 Right C 4 1 1 Trigonal pyramidal Trigonal pyramidal Sketch the molecule: H 10.43 (a) Four pairs of electrons give a tetrahedral electron geometry. The lone pair would cause lone pair-bonded pair repulsions and would have a trigonal pyramidal molecular geometry. Chapter 10 Chemical Bonding II 385 Atom Number of Electron Groups Number of Lone Pairs Molecular Geometry LeftC Center C 0 4 3 4 0 0 2 Right C 4 0 Tetrahedral Trigonal Planar Bent Tetrahedral Sketch the molecule: H (c) NH2CO2H Draw the Lewis structure and determine the geometry about each interior atom: H •• -o N. •• Number of Electron Groups Atom N 4 3 4 C O Number of Lone Pairs 1 Trigonal Pyramidal 0 2 Trigonal Planar Bent Molecular Geometry Sketch the molecule: O H \ cular Shape and Polarity Draw the Lewis structure for CC>2 and CCLj determine the molecular geometry and then the polarity. •PJ • 0 Number of electron groups on C Number of lone pairs Molecular geometry 2 0 linear •• uPI•• 4 0 tetrahedral 386 Chapter 10 Chemical Bonding II Even though each molecule contains polar bonds, the sum of the bond dipoles gives a net dipole of zero for each molecule. The linear molecular geometry of CC>2 will have bond vectors that are equal and opposite. * ^ The tetrahedral molecular geometry of CCLj will have bond vectors that are equal and have a net dipole of zero. 10.48 Draw the Lewis structure of CHsF determine the molecular geometry and then the polarity. •• • • ' F • H I C H H Number of electron groups on C 4 Number of lone pairs 0 Molecular geometry tetrahedral The molecule is tetrahedral but is polar because the C - H bond dipoles are different from the C - F bond dipoles. Because the bond dipoles are different, the sum of the bond dipoles is NOT zero. Therefore, the molecule is polar. The tetrahedral molecular geometry of Cti^F will have unequal bond vectors so the molecule will have a net dipole. 10.49 (a) PF3 - polar Draw the Lewis structure and determine the molecular geometry: The molecular geometry from Exercise 35 is trigonal pyramidal. Determine if the molecule contains polar bonds: The electronegativities of P = 2.1 and F = 4. Therefore the bonds are polar. Determine whether the polar bonds add together to form a net dipole: Because the molecule is trigonal pyramidal, the three dipole moments sum to a nonzero net dipole moment. The molecule is polar. See Table 10.2 p. 415 in text to see how dipole moments add to determine polarity. (b) SBr2 - nonpolar Draw the Lewis structure and determine the molecular geometry: The molecular geometry from Exercise 35 is bent. Determine if the molecule contains polar bonds: The electronegativities of S = 2.5 and Br = 2.8. Therefore the bonds are nonpolar. Even though the molecule is bent, since the bonds are nonpolar, the molecule is nonpolar. 388 Chapter 10 Chemical Bonding II Determine if the molecule contains polar bonds: The electronegativities of H = 2.1 and S = 2.5. Therefore the bonds are polar. Determine whether the polar bonds add together to form a net dipole: Because the molecular geometry is bent, the two dipole moments sum to a nonzero net dipole moment. The molecule is polar. See Table 10.2 p. 415 in text to see how dipole moments add to determine polarity. (a) C1O3 " - polar Draw the Lewis structure and determine the molecular geometry: Four electron pairs, with one lone pair give a trigonal pyramidal molecular geometry. Determine if the molecule contains polar bonds: The electronegativities of Cl = 3.0 and O = 3.5. Therefore the bonds are polar. Determine whether the polar bonds add together to form a net dipole: Because the molecular geometry is trigonal pyramidal, the three dipole moments sum to a nonzero net dipole moment. The molecule is polar. See Table 10.2 p. 415 in text to see how dipole moments add to determine polarity. (b) SC12- polar Draw the Lewis structure and determine the molecular geometry: Four electron pairs with two lone pairs give a bent molecular geometry. Determine if the molecule contains polar bonds: The electronegativities of S = 2.5 and Cl = 3.0. Therefore the bonds are polar. Determine whether the polar bonds add together to form a net dipole: Because the molecular geometry is bent, the two dipole moments sum to a nonzero net dipole moment. The molecule is polar. See Table 10.2 p. 415 in text to see how dipole moments add to determine polarity. (c) SC14 - polar Draw the Lewis structure and determine the molecular geometry: •• : ci : : ci — s — ici : Five electron pairs with one lone pair give a seesaw molecular geometry. Determine if the molecule contains polar bonds: The electronegativities of S = 2.5 and Cl = 3.0. Therefore the bonds are polar. Chapter 10 Chemical Bonding II 389 Determine whether the polar bonds add together to form a net dipole: Because the molecular geometry is seesaw, the four equal dipole moments sum to a nonzero net dipole moment. The molecule is polar. The seesaw molecular geometry will not have offsetting bond vectors. A (d) BrCls - nonpolar Draw the Lewis structure and determine the molecular geometry. .. ! ci CL ci: : aSix electron pairs with one lone pair gives square pyramidal molecular geometry. Determine if the molecule contains polar bonds: The electronegativity of Br = 2.8 and Cl = 3.0. The difference is only 0.2, therefore the bonds are nonpolar. Even though the molecular geometry is square pyramidal, the five bonds are nonpolar so there is no net dipole. The molecule is nonpolar. 10.52 (a) SiCl4 - nonpolar Draw the Lewis structure and determine the molecular geometry: : ci: : •• c"—si — ••ci: i 101 Four electron pairs with no lone pairs give a tetrahedral molecular geometry. .• Determine if the molecule contains polar bonds: The electronegativities of Cl = 3.0 and Si = 1.8. Therefore the bonds are polar. i Determine whether the polar bonds add together to form a net dipole: Because the molecular geometry is tetrahedral, the four equal dipole moments sum to a zero net dipole moment. The molecule is nonpolar. See Table 10.2 p. 415 in text to see how dipole moments add to determine polarity. (b) CF2C12 - polar Draw the Lewis structure and determine the molecular geometry: : ••ci I •• :•• : Four electron pairs with no lone pairs give a tetrahedral molecular geometry. Determine if the molecule contains polar bonds: The electronegativities of C = 2.5, F = 4.0, and Cl = 3.0. Therefore the bonds are polar. 390 Chapter 10 Chemical Bonding II Determine whether the polar bonds add together to form a net dipole: Even though the molecular geometry is tetrahedral, which normally yields a nonpolar molecule, the four dipole moments sum to a nonzero net dipole moment because of the different electronegativities of Cl and F. The molecule is polar. See Table 10.2 p. 415 in text to see how dipole moments add to determine polarity. (c) SeF6 - nonpolar Draw the Lewis structure and determine the molecular geometry: :F •• -/.' •• :F Six electron pairs with no lone pairs give an octahedral molecular geometry. Determine if the molecule contains polar bonds: The electronegativities of Se = 3.0 and F = 4.0. Therefore the bonds are polar. Determine whether the polar bonds add together to form a net dipole: Because the molecular geometry is octahedral, the six equal dipole moments sum to a zero net dipole moment. The molecule is nonpolar. See Table 10.2 p. 415 in text to see how dipole moments add to determine polarity. (d) - polar Draw the Lewis structure and determine the molecular geometry: •• • c- • : ••F , ••F : ..\ I S» •• s ' •• Six electron pairs with one lone pair give square pyramidal molecular geometry. Determine if the molecule contains polar bonds: The electronegativities of I = 2.0 and F = 4.0. Therefore the bonds are polar. Determine whether the polar bonds add together to form a net dipole: Because the molecular geometry is square pyramidal, the five dipole moments sum to a nonzero net dipole moment. The molecule is polar. The square pyramid structure has offsetting bond vectors in the equatorial plane, but not in the axial positions. /N y* .<•" '*',. ce Bond Theory (a) Be 2s2 No bonds can form. Beryllium contains no unpaired electrons, so no bonds can form without hybridization. Chapter 10 Chemical Bonding II 10.54 10.55 391 (b) P 3s23p3 Three bonds can form. Phosphorus contains three unpaired electrons, so three bonds can form without hybridization. (c) F 2s22p5 One bond can form. Fluorine contains one unpaired electron, so one bond can form without hybridization. (a) B 2s22p1 One bond can form. Boron contains one unpaired electron, so one bond can form without hybridization. (b) N 2s22p3 Three bonds can form. Nitrogen contains three unpaired electrons, so three bonds can form without hybridization. (c) O 2s22p4 Two bonds can form. Oxygen contains two unpaired electrons, so two bonds can form without hybridization. PH3 P [ED 3s l i t ) I (T)| (T)| 3p The unhybridized bond angles should be 90°. So, without hybridization, there is good agreement between valence bond theory and the actual bond angle of 93.3°. 10.56 SF2 s TTJ lltimimi 3s FI 3p TT] 2s F2 Umtimi 2p TT] UTUTimi 2s 2p The unhybridized bond angles should be 90°. So, without hybridization, there is not very good agreement between valence bond theory and the actual bond angle of 98.2°. 392 Chapter 10 Chemical Bonding II 10.57 2s22p2 C TTTT TTTITIT 2s22p2 C 10.58 Till I 2s 10.60 sp2 Only sp2 hybridization of this set of orbitals has a remaining p orbital to form a -IT bond. sp3 hybridization utilizes all 3 p orbitals. sp3d2 hybridization utilizes all 3 p orbitals and 2 d orbitals. sp3d sp3d hybridization utilizes an s orbital, 3 p orbitals, and d orbital. Since 5 orbitals are used, 5 hybrid orbitals form and 5 bonds can form. Hybridization utilizes an s orbital and 3 p orbitals. Four orbitals are used, so 4 hybrid orbitals form and 4 bonds can form. Hybridization utilizes an s orbital and 2 p orbitals. Three orbitals are used, so 3 hybrid orbitals form. This allows 3 cr and 1 TT bond to form for a total of 4 bonds formed. sp3 sp2 (a) CC14 Write the Lewis structure for the molecule: -8 • Use VSEPR to predict the electron geometry: Four electron groups around the central atom give a tetrahedral electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Tetrahedral electron geometry has sp3 hybridization. Sketch the molecule and label the bonds: o-C(sp3)-Cl(/>) Chapter 10 Chemical Bonding II (b) NH3 393 Write the Lewis structure for the molecule: H H N •• H Use VSEPR to predict the electron geometry: Four electron groups around the central atom give a tetrahedral electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Tetrahedral electron geometry has sp3 hybridization. Sketch the molecule and label the bonds: I Lone pair in N spi (c) OF2 Write the Lewis structure for the molecule: •• ! F •• •• O •• •• F• •• Use VSEPR to predict the electron geometry: Four electron groups around the central atom give a tetrahedral electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Tetrahedral electron geometry has sp3 hybridization. Sketch the molecule and label the bonds: I Lone pair in O s/>3 (d) CC>2 Write the Lewis structure for the molecule: Use VSEPR to predict the electron geometry: Two electron groups around the central atom give a linear electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Linear electron geometry has sp hybridization. 394 Chapter 10 Chemical Bonding II Sketch the molecule and label the bonds: <rC<sp)-0(p) 10.62 (a) CH2Br2 Write the Lewis structure for the molecule: H •• : Br•• H Use VSEPR to predict the electron geometry: Four electron groups around the central atom give a tetrahedral electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Tetrahedral electron geometry has sp3 hybridization. Sketch the molecule and label the bonds: (b) SO2 Write the Lewis structure for the molecule: •• •• o= s — o : Use VSEPR to predict the electron geometry: Three electron groups around the central atom give a trigonal planar electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Trigonal planar electron geometry has sp2 hybridization. Sketch the molecule and label the bonds: irS(p)-O(p) Lone pair in S(sp2) A - O(p) Chapter 10 Chemical Bonding II (c) JSTF-3 395 Write the Lewis structure for the molecule: •• F- " •• N- i • i • r •• Use VSEPR to predict the electron geometry: Four electron groups around the central atom give a tetrahedral electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Tetrahedral electron geometry has sp3 hybridization. Sketch the molecule and label the bonds: o-N(sp 3 )-F(p) (d) Bp3 [B Lone pair in N sp3 Write the Lewis structure for the molecule: •• :F : •• : F- F : Use VSEPR to predict the electron geometry: Three electron groups around the central atom give a trigonal planar electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Trigonal planar electron geometry has sp2 hybridization. Sketch the molecule and label the bonds: Empty p orbital o-B( 5 p 2 )-F(p) COC12 Write the Lewis structure for the molecule: :0: •••_ I—"• Use VSEPR to predict the electron geometry: Three electron groups around the central atom give a trigonal planar electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Trigonal planar electron geometry has sp2 hybridization. 396 Chapter 10 Chemical Bonding II Sketch the molecule and label the bonds: o-C(s/)-0(p) (b) BrF5 Write the Lewis structure for the molecule: F: •• IBr : F" F •• Use VSEPR to predict the electron geometry: Six electron pairs around the central atoms gives an octahedral electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Octahedral electron geometry has sp3d2 hybridization. Sketch the molecule and label the bonds: Lone pair in lAr( (c) Xep2 Write the Lewis structure for the molecule: •• : F•• •• Xe • F •• Use VSEPR to predict the electron geometry: Five electron groups around the central atom give a trigonal bipyramidal geometry. Select the correct hybridization for the central atom based on the electron geometry: Trigonal bipyramidal geometry has sp3d hybridization. Chapter 10 Chemical Bonding II 397 Sketch the molecule and label the bonds: 3 lone pairs in Xe 5p3t/ equatorial (d) 13 Write the Lewis structure for the molecule: •• : ••i - -1 : Use VSEPR to predict the electron geometry: Five electron groups around the central atom give a trigonal bipyramidal geometry. Select the correct hybridization for the central atom based on the electron geometry: Trigonal bipyramidal geometry has sp*d hybridization. Sketch the molecule and label the bonds: 3 lone pairs in 1 sp3d equatorial 10.64 (a) SO32~ Write the Lewis structure for the ion: _. 2- o- •• •• -o: Use VSEPR to predict the electron geometry: Four electron groups around the central atom give a tetrahedral electron geometry. Select the correct hybridization for the central atom based on the electron geometry: Tetrahedral electron geometry has sp3 hybridization. 402 Chapter 10 Chemical Bonding II N has four electron pairs around the atom, which is tetrahedral electron pair geometry. Tetrahedral electron pair geometry is sp3 hybridization. 10.68 C - 1 and C - 4 each have three electron groups around the atom, which is trigonal planar electron pair geometry. Trigonal planar electron pair geometry is sp2 hybridization. C - 2 and C - 3 each have four electron pairs around the atom, which is tetrahedral electron pair geometry. Tetrahedral electron pair geometry is sp3 hybridization. O - 1 and O - 2 each have four electron pairs around the atom, which is tetrahedral electron pair geometry. Tetrahedral electron pair geometry is sp3 hybridization. N has four electron pairs around the atom, which is tetrahedral electron pair geometry. Tetrahedral electron pair geometry is sp3 hybridization. Orbital Theory Is + Is constructive interference results in a bonding orbital: Bonding molecular orbital Isl Is 10.70 Is - Is destructive interference results in an antibonding orbital: Antibonding molecular orbital Destructive interference 1s -is has seven electrons. t has nine electrons. t <r tl T »> t n_^ I. AO MO AO isjfl. Jti_l- 41 IT AO MO &\s AO AO = Atomic Orbital; MO = Molecular Orbital 5 — 4 1 4 — 3 1 Bond order = - = - stable Bond order = —-— = - stable Chapter 10 Chemical Bonding II 411 Sketch the molecule and label the bonds: irC(p)-C(p) o-C(sp z )-H(s) C(sp')-C(sp3) <rC( 5 p 2 )-H(j) <rC(sp 3 )-H(s) (c) CH3SH Write the Lewis structure for the molecule: H -S•• H- H Use VSEPR to predict the electron geometry: Four electron groups around the C atom and the S atom give a tetrahedral electron geometry. Four bonding pairs of electrons around the C give a tetrahedral molecular geometry and two bonding groups and two lone pairs around the S give a bent molecular geometry. Determine if the molecule contains polar bonds: The electronegativities of C = 2.5, S = 2.5, and H = 2.1. The C - H bonds and the S - H bond will be slightly polar, and the C - S bond will be nonpolar. Determine whether the polar bonds add together to form a net dipole: In both molecular geometries the sum of the dipole moments is not zero. The molecule is polar. Select the correct hybridization for the central atom based on the electron geometry: Tetrahedral electron geometry has sp3 hybridization on both C and S. Sketch the molecule and label the bonds: o-S(sp 3 )-H(s) 2 lone paii in sp on S S(sp3)-C(jp3) crC(sp})-H« <7C(*p 3 )-H(s) senne O. H O: N H . •o•' C - 1 and C - 3 each have four electron groups around the atom. Four electron pairs give a tetrahedral electron geometry; tetrahedral electron geometry has sp3 hybridization. Four bonding pairs and zero lone pairs give a tetrahedral molecular geometry. 412 Chapter 10 Chemical Bonding II C - 2 has three electron groups around the atom. Three electron pairs give a trigonal planar geometry; trigonal planar geometry has sp2 hybridization. Three bonding pairs and zero lone pairs give a trigonal planar molecular geometry. N has four electron groups around the atom. Four electron pairs give a tetrahedral electron geometry; tetrahedral electron geometry has sp3 hybridization. Three bonding pairs and one lone pair give a trigonal pyramidal molecular geometry. O -1 and O - 2 each have four electron groups around the atom. Four electron pairs give a tetrahedral electron geometry; tetrahedral electron geometry has sp3 hybridization. Two bonding pairs and two lone pairs give a bent molecular geometry. H H (b) asparagine H H N1 1 H H IQ: d C. C3 O •• H H c= o 4 •• C - 1 and C - 3 each have four electron groups around the atom. Four electron groups give a tetrahedral electron geometry; tetrahedral electron geometry has sp3 hybridization. Four bonding groups and zero lone pairs give a tetrahedral molecular geometry. C - 2 and C - 4 each have three electron groups around the atom. Three electron groups give a trigonal planar geometry; trigonal planar geometry has sp2 hybridization. Three bonding pairs and zero lone groups give a trigonal planar molecular geometry. N - 1 and N - 2 each have four electron groups around the atom. Four electron groups give a tetrahedral electron geometry; tetrahedral electron geometry has sp3 hybridization. Three bonding groups and one lone pair give a trigonal pyramidal molecular geometry. O has four electron groups around the atom. Four electron groups give a tetrahedral electron geometry; tetrahedral electron geometry has sp3 hybridization. Two bonding groups and two lone pairs give a bent molecular geometry. H Chapter 10 Chemical Bonding II (c) 413 cysteine H H H N d ** I H <L CL O H ** H C -I and C - 3 each have four electron groups around the atom. Four electron pairs give a tetrahedral electron geometry; tetrahedral electron geometry has sp3 hybridization. Four bonding pairs and zero lone pairs give a tetrahedral molecular geometry. C - 2 has three electron groups around the atom. Three electron groups give a trigonal planar geometry; trigonal planar geometry has sp2 hybridization. Three bonding groups and zero lone pairs give a trigonal planar molecular geometry. N has four electron groups around the atom. Four electron groups give a tetrahedral electron geometry; tetrahedral electron geometry has sp3 hybridization. Three bonding groups and one lone pair give a trigonal pyramidal molecular geometry. O and S have four electron groups around the atom. Four electron groups give a tetrahedral electron geometry; tetrahedral electron geometry has sp3 hybridization. Two bonding groups and two lone pairs gives bent molecular geometry. H (a) H cytosine H N - 1 has three bonding pairs of electrons and one lone pair; four electron pairs give a tetrahedral electron geometry and sp3 hybridization. Three bonding pairs of electrons and one lone pair give a trigonal pyramidal molecular geometry. C - 2 has three bonding groups of electrons and zero lone pairs; three electron groups give a trigonal planar geometry and sp2 hybridization. Three bonding pairs of electrons give a trigonal planar molecular geometry. N - 3 has two bonding groups of electrons and one lone pair; three electron groups give a trigonal planar electron geometry and sp2 hybridization. Two bonding groups and one lone pair give a bent molecular geometry. C - 4 has three bonding groups of electrons and zero lone pairs; three electron groups give a trigonal planar geometry and sp2 hybridization. Three bonding groups of electrons give a trigonal planar molecular geometry. C - 5 has three bonding groups of electrons and zero lone pairs; three electron groups give a trigonal planar geometry and sp2 hybridization. Three bonding groups of electrons give a trigonal planar molecular geometry. 414 Chapter 10 Chemical Bonding II C - 6 has three bonding pairs of electrons and zero lone pairs; three electron groups give a trigonal planar geometry and sp2 hybridization. Three bonding groups of electrons give a trigonal planar molecular geometry. N outside the ring has three bonding groups of electrons and one lone pair; four electron groups give a tetrahedral electron geometry and sp3 hybridization. Three bonding groups of electrons give a trigonal pyramidal molecular geometry. (b) adenine NH2 (b) N - 1 has two bonding groups of electrons and one lone pair; three electron groups give a trigonal planar electron geometry and sp2 hybridization. Two bonding groups and one lone pair give a bent molecular geometry. C - 2 has three bonding groups of electrons and zero lone groups; three electron groups give a trigonal planar geometry and sp2 hybridization. Three bonding groups of electrons give a trigonal planar molecular geometry. N - 3 has two bonding groups of electrons and one lone pair; three electron groups give a trigonal planar electron geometry and sp2 hybridization. Two bonding groups and one lone pair give a bent molecular geometry. C - 4 has three bonding groups of electrons and zero lone groups; three electron groups give a trigonal planar geometry and sp2 hybridization. Three bonding groups of electrons give a trigonal planar molecular geometry. C - 5 has three bonding groups of electrons and zero lone groups; three electron groups give a trigonal planar geometry and sp2 hybridization. Three bonding groups of electrons give a trigonal planar molecular geometry. C - 6 has three bonding groups of electrons and zero lone groups; three electron groups give a trigonal planar geometry and sp2 hybridization. Three bonding groups of electrons give a trigonal planar molecular geometry. N - 7 has two bonding groups of electrons and one lone pair; three electron groups give a trigonal planar electron geometry and sp2 hybridization. Two bonding groups and one lone pair give a bent molecular geometry. C - 8 has three bonding groups of electrons and zero lone groups; three electron groups give a trigonal planar geometry and sp2 hybridization. Three bonding groups of electrons give a trigonal planar molecular geometry. N - 9 has three bonding groups of electrons and one lone pair; four electron groups give a tetrahedral electron geometry and sp3 hybridization. Three bonding groups of electrons give a trigonal pyramidal molecular geometry. N outside the ring has three bonding groups of electrons and one lone pair; four electron groups give a tetrahedral electron geometry and sp3 hybridization. Three bonding groups of electrons give a trigonal pyramidal molecular geometry. (c) thymine Chapter 10 Chemical Bonding II 415 N - 1 has three bonding pairs of electrons and one lone pair; four electron pairs give a tetrahedral electron geometry and sp3 hybridization. Three bonding pairs of electrons give a trigonal pyramidal molecular geometry. C - 2 has three bonding pairs of electrons and zero lone pairs; three electron pairs give a trigonal planar geometry and sp hybridization. Three bonding pairs of electrons give a trigonal planar molecular geometry. N - 3 has three bonding pairs of electrons and one lone pair; four electron pairs give a tetrahedral electron geometry and sp3 hybridization. Three bonding pairs and one lone pair give a trigonal pyramidal molecular geometry. C - 4 has three bonding pairs of electrons and zero lone pairs; three electron pairs give a trigonal planar geometry and sp2 hybridization. Three bonding pairs of electrons give a trigonal planar molecular geometry. C - 5 has three bonding pairs of electrons and zero lone pairs; three electron pairs give a trigonal planar geometry and sp2 hybridization. Three bonding pairs of electrons give a trigonal planar molecular geometry. C - 6 has three bonding pairs of electrons and zero lone pairs; three electron pairs give a trigonal planar geometry and sp2 hybridization. Three bonding pairs of electrons give a trigonal planar molecular geometry. C outside the ring has four bonding pairs of electrons and zero lone pairs; four electron pairs give a tetrahedral electron geometry and sp3 hybridization. Four bonding pairs of electrons give a tetrahedral molecular geometry. (d) guanine 543 9/ ^lM N - 1 has three bonding pairs of electrons and one lone pair; four electron pairs give a tetrahedral electron geometry and sp3 hybridization. Three bonding pairs of electrons give a trigonal pyramidal molecular geometry. C - 2 has three bonding pairs of electrons and zero lone pairs; three electron pairs give a trigonal planar geometry and sp2 hybridization. Three bonding pairs of electrons give a trigonal planar molecular geometry. N - 3 has two bonding pairs of electrons and one lone pair; three electron pairs give a trigonal planar electron geometry and sp2 hybridization. Two bonding pairs and one lone pair give a bent molecular geometry. C - 4 has three bonding pairs of electrons and zero lone pairs; three electron pairs give a trigonal planar geometry and sp hybridization. Three bonding pairs of electrons give a trigonal planar molecular geometry. C - 5 has three bonding pairs of electrons and zero lone pairs; three electron pairs give a trigonal planar geometry and sp2 hybridization. Three bonding pairs of electrons give a trigonal planar molecular geometry. C - 6 has three bonding pairs of electrons and zero lone pairs; three electron pairs give a trigonal planar geometry and sp2 hybridization. Three bonding pairs of electrons give a trigonal planar molecular geometry. N - 7 has two bonding pairs of electrons and one lone pair; three electron pairs give a trigonal planar electron geometry and sp2 hybridization. Two bonding pairs and one lone pair give a bent molecular geometry. C - 8 has three bonding pair of electrons and zero lone pair; three electron pairs give a trigonal planar geometry and sp2 hybridization. Three bonding pairs of electrons give a trigonal planar molecular geometry. 416 Chapter 10 Chemical Bonding H N - 9 has three bonding pairs of electrons and one lone pair; four electron pairs give a tetrahedral electron geometry and sp3 hybridization. Three bonding pairs of electrons give a trigonal pyramidal molecular geometry. N outside the ring has three bonding pairs of electrons and one lone pair; four electron pairs give a tetrahedral electron geometry and sp3 hybridization. Three bonding pairs of electrons give a trigonal pyramidal molecular geometry. 10.87 4 TT bonds; 25 a bonds; the lone pair on the Os and N - 2 occupy sp2 orbitals; the lone pairs on N - 1, N - 3, and N - 4 occupy sp3 orbitals. caffeine 10.88 5 TT bonds; 21 a bonds aspirin 'V There is rotation around the bond from C -1 to the ring and from C -1 to OH bond. There is rotation around the O - 2 to the ring bond and around the O - 2 to C - 2 bond. There is rotation around the C - 2 to C - 3 bond. The C -1 to O - 1 bond is rigid, the ring structure is rigid, and the C - 2 to O - 3 bond is rigid. 10.89 (a) Water soluble: The 4 C - OH bonds, the C = O bond, and the C - O bonds in the ring, make the molecule polar. Because of the large electronegativity difference between the C and O, each of the bonds will have a dipole moment. The sum of the dipole moments does NOT give a net zero dipole moment, so the molecule is polar. Since it is polar, it will be water soluble. (b) Fat soluble: There is only one C - O bond in the molecule. The dipole moment from this bond is not enough to make the molecule polar because of all of the nonpolar components of the molecule. The C - H bonds in the structure lead to a net dipole of zero for most of the sites in the molecule. Since the molecule is nonpolar, it is fat soluble. (c) Water soluble: The carboxylic acid function (COOH group) along with the N atom in the ring make the molecule polar. Because of the electronegativity difference between the C and O and the C and N atoms, the bonds will have a dipole moment and the net dipole moment of the molecule is NOT zero, so the molecule is polar. Since the molecule is polar, it is water soluble. (d) Fat soluble: The two O atoms in the structure contribute a very small amount to the net dipole moment of this molecule. The majority of the molecule is nonpolar because there is no net dipole moment at the interior C atoms. Because the molecule is nonpolar it is fat soluble.