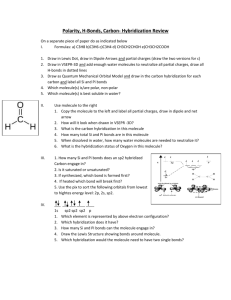
ch9 and 10 practice test 1. Which of the following covalent bonds is
advertisement

ch9 and 10 practice test 1. Which of the following covalent bonds is the most polar (highest percent ionic character)? A. Al •I 2. B. sp2 D. Si •Cl E. Si •P C. sp3 D. sp3d E. sp3d2 Which of the following solids would have the lowest melting point? A. KI 4. C. Al •Cl What is the hybridization of the central atom in ClO3– ? A. sp 3. B. Si •I B. KBr C. KCl D. KF Estimate the enthalpy change, ΔH, for the reaction 2CO + O2 → 2CO2 given the following bond energies. BE(C≡O) = 1074 kJ/mol BE(O=O) = 499 kJ/mol BE(C=O) = 802 kJ/mol A. +2380 kJ 5. D. NCl3 E. Cl2 B. 2 C. 3 D. 5 E. 8 B. Ga C. Si D. Ba E. Pb B. Cs C. P D. As E. Ge B. OF2 C. CH3Cl D. H2O E. BeCl2 The geometry of the ClF3 molecule is best described as: A. B. C. D. E. 11. C. ICl Which one of the following molecules is nonpolar? A. NH3 10. B. AsCl5 Which of the atoms listed below is the most electronegative? A. Li 9. E. -744 kJ Which of the elements listed below has the greatest electronegativity? A. Mg 8. D. -561 kJ How many covalent bonds will a nitrogen atom usually form? A. 1 7. C. +1949 kJ Which one of the following molecules has an atom with an expanded octet? A. HCl 6. B. +744 kJ distorted tetrahedron trigonal planar tetrahedral T-shaped trigonal pyramidal According to the VSEPR, theory the geometry of the atoms in the carbonate ion, CO32– is A. B. C. D. E. square planar. tetrahedral. pyramidal. trigonal planar. octahedral. Page 1 ch9 and 10 practice test 12. Which one of the following compounds does not follow the octet rule? A. NF3 13. C. PF5 D. AsH3 E. HCl The bond between which of the following pairs of atoms would be the least polar (lowest percent ionic character)? A. C •Cl 14. B. CF4 B. C •C C. C •H D. O •C E. N •C The total number of bonding electrons in a molecule of formaldehyde (H2CO) is A. 3 B. 4 C. 6 D. 8 E. 18 15. A nonpolar covalent bond (pure covalent) would form between which one of the following pairs of atoms? A. Na •Cl 16. B. H •Cl C. Li •Br D. Se •Br E. Br •Br D. sp3d E. sp3d2 What is the hybridization on the central atom in NO3– ? A. sp B. sp2 C. sp3 18. The Lewis structure for a chlorate ion, ClO3• should show ____ single bond(s), ____ double bond(s), and ____ lone pair(s). A. 2, 1, 10 19. B. 8 C. 9 D. 10 E. 13 B. XeF4 C. NF3 D. SF6 E. PF5 linear. trigonal planar. tetrahedral. bent. trigonal pyramidal. Predict the molecular geometry and polarity of the SO2 molecule. A. B. C. D. E. 23. E. 2, 1, 10 The geometry of the CS2 molecule is best described as A. B. C. D. E. 22. D. 3, 0, 10 According to VSEPR theory, which one of the following molecules should be trigonal bipyramidal? A. SF4 21. C. 2, 1, 8 Each of the three resonance structures of NO3• has how many lone pairs of electrons? A. 7 20. B. 3, 0, 9 linear, polar linear, nonpolar bent, polar bent, nonpolar None of the above. Which one of the following is most likely to be a covalent compound? A. Rb2S B. SrCl2 C. CS2 Page 2 D. CaO E. MgI2 ch9 and 10 practice test 24. The electron dot formula for AsCl3 shows: (complete) A. B. C. D. E. 25. a total of 84 electron dots three single bonds and 10 lone pairs two single bonds, one double bond, and 9 lone pairs one single bond, two double bonds, and 8 lone pairs three single bonds and one lone pair Which of the following is a useful guideline for the application of formal charges? For neutral molecules: A. a Lewis structure in which there are no formal charges is preferred. B. Lewis structures with large formal charges (+2,+3 and/or -2,-3) are preferred. C. the preferred Lewis structure is one in which positive formal charges are on the most electronegative atoms. 26. Which of the following pairs of elements would be most likely to form an ionic compound? A. B. C. D. E. 27. Cl and I Al and K Cl and Mg C and S Al and Mg Which molecule has a Lewis structure that does not obey the octet rule? A. N2O 28. B. CS2 C. PH3 D. CCl4 E. NO2 Use bond energies to estimate the enthalpy change (ΔH) for the reaction CH4 + Cl2 → CH3Cl + HCl BE(C •H) = 1074 kJ/mol BE(C •Cl) = 499 kJ/mol BE(H •Cl) = 802 kJ/mol BE(Cl •Cl) = 1074 kJ/mol A. -103 kJ 29. B. -109 kJ C. +275 kJ D. +109 kJ E. +103 kJ The Lewis dot symbol for the Ca 2+ ion is A. Ca B. • Ca• C. Ca 2+ 2+ D. Ca2+ E. Ca 30. Which of the following solids would have the highest melting point? A. NaF B. NaCl C. NaBr Page 3 D. NaI ch9 and 10 practice test 32. Predict the geometry and polarity of the CS2 molecule. A. B. C. D. E. 33. The F –Cl –F bond angles in ClF3 are A. B. C. D. E. 34. linear, polar linear, nonpolar tetrahedral, nonpolar bent, nonpolar bent, polar 90° only. 109.5° only. 120° only. 180° only. 90° and 180°. In which one of the following molecules is the central atom sp2 hybridized? A. SO2 35. B. N2O C. BeCl2 D. NF3 E. PF5 Which one of the following molecules has an atom with an incomplete octet? A. NF3 B. H2O C. AsCl3 D. GeH4 E. BF3 36. The hybridization of the phosphorus atom in the cation PH2+ is: a) b) c) d) e) sp2 sp3 dsp sp none of these 37. In the molecule C2H4 the valence orbitals of the carbon atoms are assumed to be a) b) c) d) e) not hybridized. sp hybridized. sp2 hybridized. sp3 hybridized. dsp hybridized. 38. Which of the following statements is (are) incorrect? I. II. III. IV. a) b) c) d) e) The hybridization of boron in BF3 is sp2. The molecule XeF4 is nonpolar. The bond order of N2 is three. The molecule HCN has two pi bonds and two sigma bonds. All four statements are correct. II is incorrect. I and IV are incorrect. II and III are incorrect. II, III, and IV are incorrect. Page 4 ch9 and 10 practice test 39. Atoms which are sp2 hybridized form ____ pi bond(s). a) b) c) d) e) 0 1 2 3 4 Page 5 ch9 and 10 practice test 40. The hybridization of the central atom in XeF5+ is: a) b) c) d) e) sp sp2 sp3 dsp3 d2sp3 41. The hybridization of the central atom in ClF2+ is: a) b) c) d) e) sp sp2 sp3 dsp3 d2sp3 42. The hybridization of the central atom in I3- is: a) b) c) d) e) sp sp2 sp3 dsp3 d2sp3 43. The hybridization of the central atom in O3 is: a) b) c) d) e) sp sp2 sp3 dsp3 d2sp3 44. Which of the following molecules contains a central atom with sp2 hybridization? a) b) c) Page 6 ch9 and 10 practice test d) e) 45. What hybridization is predicted for the nitrogen atom in the NO3– ion? a) b) c) d) e) sp2 sp3 sp3d sp3d2 none of these Page 7 ch9 and 10 practice test 46. Which of the following does not contain at least one pi bond? a) H2CO b) CO2 c) C 2 H4 d) C 3 H8 e) All of these (a-d) contain at least one pi bond. 47. Consider the following Lewis structure Which statement about the molecule is false? a) b) c) d) e) There are 10 sigma and 2 pi bonds. C–2 is sp2 hybridized with bond angles of 120°. Oxygen is sp3 hybridized. This molecule contains 28 valence electrons. There are some H–C–H bond angles of about 109° in the molecule. Page 8 ch9 and 10 practice test 48. Consider the following Lewis structure: What is the hybridization of the oxygen atom and carbon atoms 1, 2, and 4, respectively? Option 1: Option 2: Option 3: Option 4: Option 5: a) b) c) d) e) O C–1 3 sp3 sp sp3 sp sp sp2 sp2 sp3 sp sp3 Option 1 Option 2 Option 3 Option 4 Option 5 C–2 sp sp sp sp2 sp2 C–4 sp2 sp sp2 sp3 sp 49. The following statements concern molecules that require resonance. Which is true? a) b) c) d) e) The pi bonding is most clearly delocalized. The sigma bonding is most clearly delocalized. Both the sigma and pi bonding are delocalized. The benzene molecule is best described by the MO theory. The benzene molecule is best described by the localized electron model. 50. Consider the benzene molecule. Which of the following statements about the molecule is false? a) b) c) d) e) All six C ⎯ C bonds are known to be equivalent. Each carbon atom is sp2 hybridized. The localized electron model must invoke resonance to account for the six equal C ⎯ C bonds. It has delocalized pi bonding in the molecule. The pi bonds of carbon involve sp2 orbitals. Page 9 ch9 and 10 practice test Answer Key for Test "ch 9 and 10 test.tst", 2/17/2010 No. in Q-Bank 9-33 10-32 9-20 9-66 9-63 9-50 9-28 9-25 10-18 10-6 10-7 9-59 9-37 9-44 9-31 10-29 9-23 9-47 9-56 10-9 10-5 10-23 9-5 9-43 9-51 9-4 9-64 9-69 9-14 9-18 9-22 10-24 10-16 10-30 9-62 No. on Test 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 Correct Answer C C A D B C C C E D D C B D E B D D B E A C C B A C E A D A E B E A E 36.ANS:a) sp2 PAGE: 9.1 37ANS: c) sp2 hybridized. PAGE: 9.1 38ANS: a) All four statements are correct. PAGE: 9.1 39ANS:b) 1 PAGE: 9.1 40ANS: e) d2sp3 PAGE: 9.1 Page 10 ch9 and 10 practice test 41ANS: c) sp3 PAGE: 9.1 42ANS:d) dsp3 PAGE: 9.1 43ANS: e) sp2 PAGE: 9.1 ANS: b) PAGE: 9.1 44 45ANS: a) sp2 PAGE: 9.1 46ANS:d) C 3 H8 PAGE: 9.1 47ANS: c) Oxygen is sp3 hybridized. PAGE: 9.1 48ANS: a) Option 1 PAGE: 9.1 49ANS: a) The pi bonding is most clearly delocalized. PAGE: 9.5 50ANS: e) The pi bonds of carbon involve sp2 orbitals. PAGE: 9.5 Page 11