Chapter 14b: Other pathways of carbohydrate metabolism
advertisement

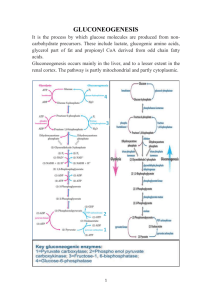
Chapter-14b 1 Takusagawa’s Note Chapter 14b: Other Pathways of Carbohydrate Metabolism 1. GLUCONEOGENESIS - is the biosynthesis of glucose from non-carbohydrate precursors at liver and kidney (very small amount). - Most excess glucose from diet is stored in liver as glycogen. - However glycogen stored in liver is only a half day supply of glucose to the brain under fasting or starvation conditions. - Liver can send glucose to various tissues, but not ATP. - Thus, when fasting, most glucose needs must be obtained by gluconeogenesis at liver, and the produced glucose is sent to various tissues through bloodstream. A. Gluconeogenesis Pathway The first step of gluconeogenesis is conversion of various compounds to oxaloacetate - Glycolysis products - lactate & pyruvate - Citric acid cycle intermediates are converted to Oxaloacetate. - Carbon skeletons of most amino acids 1 Chapter-14b - 2 Takusagawa’s Note Leucine and lysine are not converted to oxaloacetate, these amino acids break down to acetylCoA, likewise fatty acids. In plants, acetyl-CoA can convert to oxaloacetate by glyoxylate cycle, but not in animals. Gluconeogenesis use the same glycolytic enzymes, except for hexokinase, phosphofructokinase and pyruvate kinase Glycolysis pathway - Hexokinase (HK) Glucose (glucose→ glucose-6-phosphate) ↓ hexokinase (HK) G6P - Phosphofructokinase (PFK) ↓ phosphoglucose isomerase (PGI) (fructose-6-phosphate → F6P fructose-1,6-bisphosphate) ↓ phosphofructokinase (PFK) FBP - Pyruvate kinase (PK) ↓ aldolase (PEP → pyruvate) DHAP/GAP ↓ triose phosphate isomerase (TIM) GAP ↓ glyceraldehyde-3-phosphate dehydrogenase BPG (GAPDH) ↓ phosphoglycerate kinase (PGK) 3PG ↓ phosphoglycerate mutase (PGM) 2PG ↓ enolase PEP ↓ pyruvate kinase (PK) Pyruvate ↓ pyruvate dehydrogenase Oxaloacetate → Citrate - These enzymes catalyze reactions with large negative free energy changes (∆G < 0, exergonic reaction). Energy Glucose ∆G ≅ 0 ∆G < 0 G6P→F6P ∆G ≅ 0 ∆G < 0 FBP→DHAP/GAP→GAP→BPG→3PG→2PG→PEP ∆G < 0 Pyruvate Glycolysis Products - Thus, these processes in gluconeogenesis must take different pathways. 2 Takusagawa’s Note 3 Chapter-14b Pyruvate is converted to oxaloacetate before conversion to phosphoenolpyruvate (PEP) - As described in the previous page, pyruvate is not directly converted to PEP since the reverse reaction is endergonic. - Thus the pyruvate-PEP conversion takes the different pathway. - Two enzymes are involved. 1. Pyruvate carboxylase driven by ATP catalyzes formation of oxaloacetate from pyruvate and HCO3-. 2. PEP carboxykinase (PEPCK) catalyzes decarboxylation of oxaloacetate to PEP by using GTP hydrolysis energy. Pyruvate carboxylase has a biotin prosthetic group - Biotin is a CO2 carrier, and is an essential human nutrient. - Biotin is covalently bound to the enzyme by amide linkage between its carboxyl group and εamino group of Lys residue of the enzyme. - Biotin is therefore at the end of 14 Å long flexible arm so that CO2 molecule can be transported between the two relatively separated positions. O C HN NH O C O S (CH2)4 H3N C (CH2)4 CH O - NH Biotin Lys residue O O C N - NH O O S - 14 A long flexible arm C (CH2)4 C N H O C (CH2)4 CH NH Carboxybiotinyl-enzyme Note: Biotinyl-enzyme is similar to lipoic acid + Lys-enzyme = lipoamide-enzyme, i.e., Pyruvate dehydrogenease multi-enzyme complex (Chapter 15). 3 Chapter-14b 4 Takusagawa’s Note Pyruvate carboxylase reaction - occurs in two phases. 1. Carboxylation of biotin-Enzyme - ATP reacts with carbonate (HCO3-) to produce carboxyphosphate and ADP. The hydrolysis of (ATP → ADP + Pi) provides free energy for carboxylation of biotin. - Carboxyphosphate decomposes to Pi and CO2. - CO2 carboxylates biotin to produce the very reactive carboxybiotinyl-enzyme. 2. The activated carboxyl group is transferred from carboxybiotin to pyruvate to form oxaloacetate. 4 Takusagawa’s Note 5 Chapter-14b Oxaloacetate can be considered as “activated” pyruvate with CO2 - Because oxaloacetate is synthesized with ATP hydrolysis energies from pyruvate and CO2. - Thus, it carries the ATP hydrolysis energy with CO2. O - O C O + CH3 C O C O C O CH2 O C - O O Oxaloacetate C - O Pyruvate + CO2 Acetyl-CoA regulates pyruvate carboxylase - In general, [oxaloacetate] is very low in cells. Thus oxaloacetate synthesis increases the citric acid cycle activity, i.e., increases acetyl-CoA demand. - Thus, acetyl-CoA is a powerful allosteric activator of pyruvate carboxylase. - Gluconeogenesis only occurs when the citric acid cycle is inhibited by excess of ATP and/or NADH. PEP carboxykinase (PEPCK) - catalyzes the GTP-driven decarboxylation of oxaloacetate to form PEP. 5 Takusagawa’s Note 6 Chapter-14b Gluconeogenesis requires metabolite transport between mitochondria and cytosol - Since gluconeogenesis is carried out in cytosol, the mitochondrial products, PEP and oxaloacetate, must be transported to cytosol. - PEP is transported through the specific membrane transport proteins. - Oxaloacetate does not have such transport proteins. - Thus, oxaloacetate is transported by the malate-aspartate shuttle (remember: the cytosol NADH transport in Chapter 17). - There are two routes. Mitochondrion Cytosol aspartate aminotransferae Route-1: Oxaloacetate aspartate aminotransferae Aspartate Oxaloacetate Aspartate malate dehydrogenease Route-2: Oxaloacetate NADH + H+ malate dehydrogenease Malate Oxaloacetate Malate NAD+ NAD+ NADH + H+ PEP PEP Gluconeogenesis - Since NADH is utilized for gluconeogenesis, Route-2 is the major route since it transports not only oxaloacetate but also NADH at the same time. If lactate is precursor, then both routes can be used since oxidation of lactate to pyruvate produces NADH at cytosol (lactate + NAD+ ↔ pyruvate + NADH + H+). The other two highly unfavorable reverse reactions are bypassed by hydrolytic reactions - The other two highly unfavorable reverse reactions are: - PFK and hexokinase reactions whose reverse reaction produce ATPs, i.e., FBP + ADP + Pi → F6P + ATP G6P + ADP + Pi → Glucose + ATP - Instead the above reactions, FBP and G6P are directly hydrolyzed, and releasing Pi in exergonic process, i.e., FBP + H2O → F6P + Pi -- [1] catalyzed by fructose-1,6-bisphosphatase (FBPase) G6P + H2O → Glucose + Pi -- [2] catalyzed by glucose-6-phosphatase (only present at liver and kidney). 6 Chapter-14b - - - 7 Takusagawa’s Note Gluconeogenesis is accomplished by avoiding three energetically unfavorable reverse reactions and by expensing hydrolysis of 4ATP, 2GTP and 2NADH per glucose. 2(Pyruvate) + 4ATP + 2GTP + 2NADH + 4H+ + 6H2O → Glucose + 2NAD+ + 4ADP + 2GDP + 6Pi Note: Glycolysis produces 2ATP and 2NADH per glucose: Glucose + 2NAD+ + 2ADP + 2Pi → 2(Pyruvate) + 2ATP + 2NADH + 4H+ + 2H2O The overall reaction (a couple of glycolysis and gluconeogenesis) wastes 2ATP and 2GTP: 2ATP + 2GTP + 4H2O → 2ADP + 2GDP + 4Pi Glycolysis Gluconeogenesis 7 8 Chapter-14b Takusagawa’s Note B. Regulation of gluconeogenesis - If glycolysis and gluconeogenesis were to proceed in uncontrolled manner, ATP and GTP would be wasted. - Thus, glycolysis and gluconeogenesis pathways are reciprocally regulated. - Three independent pathways are regulated: 1. Glucose ↔ G6P (HK/glucose-6-phosphatase) 2. F6P ↔ FBP (PFK/FBPase) 3. PEP ↔ Pyruvate (PK/pyruvate carboxylase-PEPCK) - Dominant mechanisms are: 1. Allosteric interactions (by F2,6P). 2. cAMP-dependent covalent modifications (cAMP levels are controlled by glucagon and other hormones). Important allosteric effector is fructose-2,6-bisphosphate (F2,6P) - (see Chapter 14, page 18-19). - F2,6P activates PFK (glycolysis) and inhibits FBPase. - [F2,6P] is controlled by two enzyme activities: 1. Phosphofructokinase-2 (PFK-2) --- synthesis of F2,6P. 2. Fructose bisphosphatase-2 (FBPase-2) --- breakdown of F2,6P. - Note: Phosphorylation inactivates liver PFK-2 and activates liver FBPase-2. Phosphorylation activates heart PFK-2 and inactivates heart FBPase-2. PFK-2 and FBPase-2 are not directly involved in glycolysis and gluconeogenesis. - Low levels of [glucose] results in hormonal activation of gluconeogenesis through regulation of [F2,6P]. Low blood [glucose] ↓ Increased glucagon secretion from panceas ↓ In Liver - Increased [cAMP] ↓ Increased enzyme phosphorylation ↓ Activation of FBP-2 and inactivation of PFK-2 ↓ Decreased [F2,6P] ↓ Inhibition of PFK and activation of FBPase ↓ Increased gluconeogenesis Activation of gluconeogenesis in liver involves inhibition of glycolysis at the level of liver PK. Liver PK is inhibited allosterically by alanine (precursor of pyruvate) and phosphorylation. Muscle PK (isozyme of liver PK) is not subject to these control. 8 Takusagawa’s Note 9 Chapter-14b C. The Cori Cycle - is discovered by Carl and Gerti Cori. - Muscle contraction is powered by hydrolysis of ATP. - ATP is regenerated either: 1. through oxidative phosphorylation in mitochondria of slow twitch (red) muscle fibers (aerobic pathway, slow ATP production), or 2. by homolactic fermentation (pyruvate → lactate) in fast-twitch (white) muscle fiber (anaerobic pathway, fast ATP production). - Lactate generated by homolactic fermentation is transferred via bloodstream to the liver, then converted to pyruvate, then put into gluconeogenesis to produce glucose. - The produced glucose is transferred from liver to muscle cells via bloodstream, and generate ATP. - This cycle is called Cori cycle. - One cycle of Cori cycle wastes 2ATP and 2GTP per glucose. - This Cori cycle is ATP/GTP-consuming glycolysis/gluconeogenesis “futile cycle”, but not in the same cell. - Thus, muscle cells can obtain ATP quickly by the Cori cycle. Gluconeogenesis Glycolysis Muscle Liver blood Glucose ADP + GDP +P i Lactate Glycogen P i + ADP glycogenolysis and glycolysis gluconeogenesis ATP + GTP Glucose blood 9 ATP Lactate Chapter-14b 10 Takusagawa’s Note 2. GLYOXYLATE PATHWAY - Glyoxylate pathway occurs only in plants, but not in animals. - converts acetyl-CoA to glyoxylate which condenses with malate to yield oxaloacetate. - This oxaloacetate is used for production of glucose in gluconeogenesis. - Thus, plants can convert acetyl-CoA to glucose by glyoxylate pathway. Reaction pathway 1. Mitochondrial oxaloacetate is converted to aspartate, and transported to glyoxysome. 2. Aspartate in glyoxysome is reconverted to oxaloacetate. 3. Oxaloacetate is condensed with acetylCoA to form citrate, which is isomerized to isocitrate. 4. Isocitrate is cleaved to succinate and glyoxylate by glyoxysomal isocitrate lyase. 5. Glyoxylate is condensed with acetyl-CoA to produce malate by glyoxysomal malate synthase. 6. Malate is transported in cytosol, and is oxidized by the cytosol malate dehydrogenase to yield oxaloacetate which enters gluconeogenesis to produce glucose. 7. Succinate is transported to mitochondrion and is entered into the citric acid cycle. Fig. 21-10. 10 - Takusagawa’s Note 11 Chapter-14b In the citric acid cycle, conversion of isocitrate (C6) to succinate (C4) produces two CO2. On the other hand, in the glyoxylate pathway, conversion of isocitrate to succinate produces glyoxylate. Thus, glyoxylate pathway results in the net conversion of acetyl-CoA to glyoxylate instead of 2CO2. O H3C C SCoA citric acid cycle glyoxylate pathway O 2CO2 2NADH + GTP - H C COO Glyoxylate Gluconeogenesis Oxidative phosphorylation - The net reaction of glyoxylate pathway is: 2(Acetyl-CoA) + 2NAD+ + 2FAD → Oxaloacetate(C4) + 2CoA + 2NADH + FADH2 + 2H+ - The net reaction of citric acid cycle is: 2(Acetyl-CoA) + 6NAD+ + 2FAD + 2GDP + Pi → 4CO2 + 2CoA + 6NADH + 2FADH2 + 2GTP + 6H+ - Plants (germinating seeds) use the glyoxylate pathway to convert their stored triacylglycerols to glucose. Story of Jack and Bean Stalk Cellulose (Glucose)n Seed Triacylglycerol Short time Glucose Note: Acetyl-CoA is not a precursor of gluconeogenesis in animal cells, i.e., animals cannot produce glucose from acetyl-CoA (← fatty acid ← triacylglycerol). 11 Chapter-14b 12 Relation between the citric acid cycle and glyoxylate pathway Two L-malates are produced and one enters gluconeogenesis to produce glucose and the other stays in the citric acid cycle. 12 Takusagawa’s Note Chapter-14b 13 Takusagawa’s Note 3. BIOSYNTHESIS OF OLIGOSACCHARIDES AND GLYCOPROTEINS Glycosyl donors - Glycosyl donors are nucleotide-sugars (such as UDP-glucose) which are synthesized by condensation between monosaccharide-phosphate (such as G1P) and nucleosidetriphosphate (such as UTP). - Nucleotide-sugars contain a high energy bond between C1’ and OP. - Energy from hydrolysis of high energy bond is used to formation of glycosidic bond (∆G°’= 16 kJ/mol) between the two sugars. - Oligosaccharide biosyntheses are catalyzed by glycosyl transferases. A. Lactose synthesis - The donor sugar is UDP-galactose and acceptor sugar is glucose. - The reaction is catalyzed by lactose synthase. 13 Takusagawa’s Note 14 Chapter-14b B. Glycoprotein synthesis - Proteins destined for secretion, incorporation into membranes, or localization inside membranous organelles contain carbohydrates and therefore classified as glycoproteins. - The oligosaccharide portions of glycoproteins are classified into three groups. 1. N-linked oligosaccharides - linked by β-N-glycosidic bond to Asn in Asn-X-Ser/Thr, X≠ Pro. - N-linked oligosaccharides are constructed on dolichol. CH3 H CH2 C O CH3 CH CH2 CH2 C CH2 CH2 O P O P O - Isoprene unit - n O O carbohydrate - O Saturated α-isoprene unit Dolichol (is hydrophobic, thus stays inside membrane = Ancho All processes are carried out at rough endoplasmic reticulum. 14 Chapter-14b 15 Takusagawa’s Note The pathway of dolichol-PP-oligosaccharide synthesis 1. A dolichol phosphate anchors into the membrane of endoplasmic reticulum with the phosphate group in cytosol. 2. A oligosaccharide is constructed on the dolichol phosphate. 3. The dolichol is flipped so that the oligosaccharide is moved from the cytosol side to the rumen side. 4. Monosaccharides are attached on the other phosphate dolichols and are translocated into rumen. 5. These monosaccharides are utilized to complete the oligosaccharide construction on the dolichol. 6. After completion of oligosaccharide construction, the mature oligosaccharide is transferred to the newly synthesized polypeptide at an Asn residue. 15 Chapter-14b 16 Takusagawa’s Note 2. O-linked oligosaccharides - are linked by α-O-glycosidic bond to Ser or Thr. - are posttranslationally formed in Golgi apparatus. - Monosaccharide units are added one by one on a completed polypeptide chain. Mature polypeptide chain 3. GPI-linked proteins - Glycosylphosphatidylinositol (GPI) groups are anchors of variety of proteins to the exterior surface of eukaryotic plasma membrane. - GPI core is synthesized inside of lumenal side of the ER membrane from phosphatidylinositol, UDP-GlcNAc, and dolichol-P-mannose. 16 Chapter-14b 17 Takusagawa’s Note 4. PENTOSE PHOSPHATE PATHWAY - Cells have two currencies: 1. Energy currency (ATP) 2. Reducing power currency (NADPH) - - Although NADH and NADPH are chemically resemblance, those are not metabolically interchangeable. - NADH participates in utilizing the free energy of the oxidation of (NADH → NAD+) to synthesize ATP. - NADPH involves in utilizing the free energy of the oxidation of (NADPH → NADP+) to carry out endergonic reductive biosynthesis. In cells, - [NAD+] / [NADH] ≈ 1000 (favors metabolite oxidation). AH + NAD+ → A+ + NADH [oxidation reaction] - [NADP+] / [NADPH] ≈ 0.01(favors metabolite reduction). [reduction reaction] B+ + NADPH → BH + NADP+ - NADPH is generated by the oxidation of G6P via an alternative pathway to glycolysis, pentose phosphate pathway. - ~30% of glucose oxidation in liver occurs via the pentose phosphate pathway. The pentose phosphate pathway has three stages 1. Oxidative reactions, which yield NADPH and ribulose-5-phosphate (Ru5P) 3G6P + 6NADP+ + 3H2O → 6NADPH + 6H+ + 3CO2 + 3Ru5P 2. Isomerization and epimerization reactions, which transfer Ru5P to ribose-5-phosphate(R5P) [is an essential precursor in the biosynthesis of nucleotides] and xylulose-5-phosphate (Xu5P). 3Ru5P ↔ R5P + 2Xu5P 3. A series of C-C bond cleavage and formation reactions, which convert (R5P + 2Xu5P) to fructose-6-phosphate (F6P) and glyceraldehyde-3-phosphate (GAP). R5P + 2Xu5P ↔ 2F6P + GAP - Overall reaction of pentose phosphate pathway: 3G6P + 6NADP+ + 3H2O ↔ 6NADPH + 6H+ + 3CO2 + 2F6P + GAP Note: Ribulose-5-phosphate (Ru5P)--- ketose Ribose-5-phosphate (R5P)------ aldose 17 Chapter-14b 18 Pentose phosphate pathway ± C2 6: C5 + C5 → C 7 + C3 ± C3 7: C7 + C3 → C4 + C6 ± C2 8: C4 + C5 → C 6 + C3 18 Takusagawa’s Note Chapter-14b 19 Takusagawa’s Note A. Oxidative reactions of NADPH production - Only the first three reactions of the pentose phosphate pathway are involved in NADPH production. 1. Glucose-6-phosphate dehydrogenase catalyzes net transfer of a hydride ion to NADP+ from C1 of G6P to form 6-phosphoglucono-δ-lactone. - This enzyme is the rate-determing enzyme of the pentose phosphate pathway. This enzyme is the rate-determining enzyme of pentose pathway. 2. 6-Phosphogluconolactonase increases the rate of hydrolysis of 6-phosphoglucono-δ-lactone to 6-phosphogluconate. 3. Phosphogluconate dehydrogenase catalyzes the oxidative decarboxylation of 6phosphogluconate to Ru5P and CO2. This oxidation reaction reduces the second NADP+ to NADPH. 19 Chapter-14b B. - 20 Takusagawa’s Note Isomerization and epimerization of ribulose-5-phosphate The produced Ru5P must subsequently be converted to R5P or Xu5P for further use. Ru5P is converted to R5P by ribulose-5-phosphate isomerase. R5P is an essential precursor in the biosynthesis of nucleotide. Ru5P is also converted to Xu5P by ribulose-5-phosphate epimerase. If more R5P is formed than the cell needs, the excess, along with Xu5P, is converted to F6P and GAP which are glycolytic intermediates. 20 Chapter-14b 21 C. Carbon-Carbon bond cleavage and formation reactions - Excess of pentose cannot enter the glycolysis, gluconeogenesis or pentose phosphate pathway, thus they are converted to hexoses (F6P) and trioses (GAP) by C-C bond cleavage and formation reactions. - Two enzymes are involved: 1. Transketolase that catalyzes the transfer of C2 units (-CO-CH2OH). - Transketolase has a thiamine pyrophosphate cofactor (TPP). - TPP forms a covalent adduct with Xu5P, Xu5P-TPP. - By releasing GAP, Xu5P-TPP becomes dihydroxyethyl-TPP. - Then the dihydroxyethyl group (C2 unit) is transferred to R5P at C1 to produce sedoheptulose-7-phosphate (S7P). 1. E·TPP + Xu5P → E·TPP-Xu5P 2. E·TPP-Xu5P → E·TPP-C2 + GAP 3. E·TPP-C2 + R5P → E·TPP-S7P 4. E·TPP-S7P → E·TPP + S7P - The overall reaction is to transfer a C2 unit from Xu5P to R5P to yield GAP and S7P: Xu5P + R5P → GAP + S7P C C5 + C5 2 → C3 + C7 21 Takusagawa’s Note Chapter-14b 22 2. Transaldolase catalyzes the transfer of C3 units (-CHOH-CO-CH2OH). - Transaldolase catalyzes the transfer of C3 unit from S7P to GAP yielding erythrose-4-phosphate (E4P) and F6P. - This is an aldol cleavage reaction. - The enzyme has an essential Lys residue whose ε-amino group forms a Schiff base with the carbonyl group of S7P. 1. E-Lys + S7P → E-Lys-S7P 2. E-Lys-S7P → E-Lys-C3 + E4P 3. E-Lys-C3 + GAP → E-Lys-F6P 4. E-Lys-F6P → E-Lys + F6P - The overall reaction is to transfer a C3 unit from S7P to GAP to yield E4P and F6P: S7P + GAP → E4P + F6P C C7 + C3 3 → C4 + C6 22 Takusagawa’s Note Chapter-14b 23 Takusagawa’s Note A second transketolase reaction yields GAP and a second F6P - The C2 unit of Xu5P is transferred to E4P to form GAP and F6P. - The third phase of the pentose phosphate pathway is therefore transformation of two molecules of Xu5P and one of R5P to form two molecules of F6P and one molecule of GAP. 2Xu5P + R5P → 2F6P + GAP - The summary of the third phase reactions is: 1st: C5 + C5 ↔ C7 + C3 [C2 transfer by transketolase] 2nd: C7 + C3 ↔ C6 + C4 [C3 transfer by transaldolase] 3rd: C5 + C4 ↔ C6 + C3 [C2 transfer by transketolase] Sum: 3C5 ↔ 2C6 + C3 D. Control of the pentose phosphate pathway - Principal products of pentose phosphate pathway are: NADPH and R5P (processor of nucleotide biosynthesis). - The excess of pentoses are converted to F6P and GAP which can enter either glycolysis or gluconeogenesis to the pentose phosphate pathway. - In the latter case, one G6P molecule can be converted to 6CO2 via 6 cycles of the pentose phosphate pathway and gluconeogenesis, and produces 12NADPH molecules. - Proof: 6G6P → 4F6P + 2GAP + 12NADPH + 6CO2 ⇓ 5G6P ⇐ 4G6P + G6P ____________________________________________________________ G6P → 12NADPH + 6CO2 - The pentose phosphate pathway and thus the rate of NADPH production are controlled by the rate of the glucose-6-phosphate dehydrogenase reaction (first committed step with ∆G = -17.6 kJ/mol in liver). [NADP + ] + - The enzyme is regulated by [NADP ], i.e., the substrate availability. ≈ 0.01 [NADPH] Example of NADPH usage - Erythrocyte membrane integrity requires a plentiful supply of reduced glutathione (GSH) to eliminate H2O2 and R-O-O-H. - These hydroperoxides are toxic products that can react with double bonds in the fatty acid residues, and consequently the C-C bonds in fatty acids are cleaved, thereby damaging the erythrocyte membrane. - These hydroperoxides must be eliminated through the action of glutathione peroxidase. glutathione peroxidase 2GSH + R-O-O-H → GSSG + ROH + H2O - Then GSH is subsequently regenerated by the NADPH reduction of GSSG. glutathione reductase GSSG + NADPH → 2GSH + NADP+ - Thus, a steady supply of NADPH is vital for erythrocyte membrane integrity. 23