Exam 3 - Chemistry Courses: About
advertisement

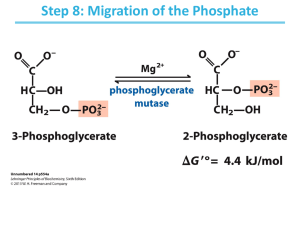
C483 Summer 2015 Exam 3 Name ______________________________________________ 1. 20 pts Fill in the blanks (2 points each) A. The total energy cost of getting one molecule through the energy-investment phase of glycolysis is _________________________. B. Once pyruvate is formed in glycolysis, it can be made into _______________ under anaerobic conditions, or __________________ under aerobic conditions in preparation for the citric acid cycle. C. If a molecule of glucose is converted into two pyruvate molecules, which are then converted back to glucose through gluconeogenesis, the total cost would be ____________ ATP equivalents. D. ____________ and _________________ are the two metabolically useful products of the oxidative phase of the pentose phosphate pathway. E. A transketalase enzyme requires the cofactor ________________ in order to transfer an acyl unit. F. If one molecule of acetyl CoA runs through the citric acid cycle, _________ ATP equivalents are formed at the substrate phosphorylation level, and __________ NADH are produced. G. Increasing the concentration of citric acid cycle intermediates increases ________ through the pathway. H. According to the Binding Change mechanism, ATP synthase adopts a ___________ conformation to form the ATP high energy bond and a __________ conformation to release the newly formed ATP molecule. I. An ________________ agent is toxic because it separates the protonmotive force from its ability to produce ATP. J. The P:O ratio is the number of __________________ relative to the number of oxygen atoms reduced. 2. 10 points True or false (1 point each) A. ______________ Although aldolase catalyzes a reaction with a large positive standard free energy, the reaction is spontaneous under cellular conditions. B. ______________ Gluconeogenesis occurs primarily in muscle tissue. C. ______________ Glycogen is broken down into glucose through a hydrolysis reaction to ensure that it is thermodynamically favorable. D. _____________ Five carbon sugars are interconverted to 3 and 7 carbon sugars by a transketalase and 3 and 7 carbon sugars are interconverted to 4 and 6 carbon sugars by a transaldolase. E. ______________ The two carbon dioxide forming reactions of the citric acid cycle both employ decarboxylase enzymes. F. ______________ - ketogluterate dehydrogenase is more similar to isocitrate dehydrogenase than pyruvate dehydrogenase in its chemical mechanism. G. ____________ Carboxylase enzymes generally have biotin as a cofactor and require ATP. H. ____________ NADH, citrate, and ADP inhibit the citric acid cycle. I. ____________ High energy electrons that enter the electron transport chain through Complex II pump less protons than high energy electrons entering the electron transport chain directly into the Q pool. J. _____________ In mammals, fatty acids cannot be used to make net glucose, but the glyoxylate pathway in plants allows fats to be made into net glucose. 3. (20pts) Short answer (5 points each) A. Write the net equation for ONE of the following: glycolysis starting from glucose, or gluconeogenesis starting from pyruvate. (You do not need to balance the water and protons, but need stoichiometry numbers otherwise, including necessary stoichiometric cofactors.) B. Use data provided at the end of the exam to show that the standard free energy change for the reaction catalyzed by phosphoglycerate kinase is -18.9 kJ/mol. C. Use data provided at the end of the exam to calculate the standard reduction potential for the reduction of pyruvate by NADH. D. Draw a schematic of a mitochondrion and label the membranes and spaces. Where does the citric acid cycle take place? Where is ATP synthesized during oxidative phosphorylation? Indicate the direction of the proton gradient established by respiration. Problems (10pts each) 4. For each of the following reactions, indicate A. irreversible/near equilibrium, B. pathway/process, C. necessary cofactors (or write none.) Reaction Pyruvate oxaloacetate Two 5-carbon sugars 3 carbon and 7 carbon sugars Fumarate malate Citrate isocitrate Irreversible/ near equilibrium Pathway/process 5. Provide an enzyme catalyzed reaction mechanism for one of the following two reactions. A. Glyceraldehyde-3-phosphate + inorganic phosphate 1,3-bisphosphoglycerate B. Fructose-1,6-bisphosphate dihydroxyacetone phosphate + glyceraldehyde-3-phosphate 6. How much ATP can be obtained by the cell from the complete oxidation of one mole of glucose under aerobic conditions? Explain how you got this number, and how it can be affected by transport systems. Compare this value with the ATP obtained when glucose is converted to lactate. 7. Draw a simple schematic of the electron transport chain, including all complexes and the Q pool. Indicate where the initial high energy electrons (what compound) are put into the system, and where the electrons are finally donated (which molecule)? A synthetic compound has been developed which is able to donate two electrons directly to Complex IV, producing a proton gradient. What is the theoretical P:O ratio of this compound? Show all work. 8. The citric acid cycle A. Reaction 8 and reaction 1 of the citric acid cycle can be considered to be coupled because the exergonic first step drives regeneration of oxaloacetate in step 8, which has a positive standard free energy. Chemically speaking, why is step one energetically favorable? What is the chemical basis for reactions 1 and 8 being coupled? B. Oxaloacetate labeled at C4 with 14C is added to a suspension of respiring mitochondria. What is the fate of the labeled carbon? C. Acetyl-CoA is labeled at C1 with 14C and added to a suspension of respiring mitochondria. Explain why all the label is lost after two rounds of the citric acid cycle. 9. (10pts Case study) During even mild exertion, individuals with McArdle’s disease experience painful muscle cramps due to a genetic defect in glycogen phosphorylase, the enzyme that breaks down glycogen. (The liver shows no swelling and appears normal.) Yet the muscles in these individuals contain normal amounts of glycogen. What did these observations concerning muscle tissue tell researchers about the pathways for glycogen synthesis and degradation? A patient with McArdle’s disease performs anaerobic exercise as long as he is able. Blood is withdrawn from the patient every few minutes during the exercise period and tested for lactate. The patient’s samples are compared with a healthy control in the figure below. Explain the metabolic reason for the difference in the shape of the curves. Does a patient with McArdle’s disease suffer from hypoglycemia (low blood sugar), hyperglycemia, or neither? Explain. [𝑋]𝑓𝑖𝑛𝑎𝑙 G = RT ln[𝑋]𝑖𝑛𝑖𝑡𝑖𝑎𝑙 + ZF R = 8.314 J/ mol . K F = 96,485 J/V . mol G = -nFEo’